Heating during chemical reactions of carbon black
Back to Subsection DG chemicals
Oxidative treatment of carbon black
Oxygen containing functional groups on the surface of carbon blacks strongly influence their application properties. High contents of volatile components, i.e. high concentrations of surface oxides, decrease the vulcanisation rate and improve the flow characteristics of inks. The gloss of lacquers and coatings is increased, the colour tone is shifted from brownish to bluish, and jetness (degree of blackness) often increases. Due to the production conditions, only gas blacks (and channel blacks) are covered to a certain extent with acidic surface oxides. Furnace blacks contain only small amounts of oxygen in the form of basic surface oxides. To amend their colour properties, some pigment blacks are post-treated by oxidation on a commercial scale. Depending on the oxidising agent and the reaction conditions selected, different types of surface oxides are formed in varying quantities. The simplest method of oxidising the carbon black surface is by post-treating it with air at 350 – 700 °C (see Figure 1).
Figure 4.6: Equipment for the oxidative treatment of carbon black in a fluidised bed [47, InfoMil, 2002]
However, the degree of oxidation is limited. Higher contents of surface oxides and better
process control are achieved with nitric acid, mixtures of NO2 and air, or ozone. Also aqueous
solutions such as sodium hypochlorite solutions may be used as oxidising agents. As a rule, all
strongly oxidising agents may be used, either as a gas or in solution. Most surface oxidation
processes of carbon black are carried out at elevated temperatures. Oxidised carbon blacks may
contain up to 15 wt-% oxygen. They are strongly hydrophilic, and some of them form colloidal
solutions spontaneously in water. In polar printing ink systems, and in lacquers and coatings, a
better wettability and dispersibility is achieved through surface oxidation, thus reducing binder
consumption.
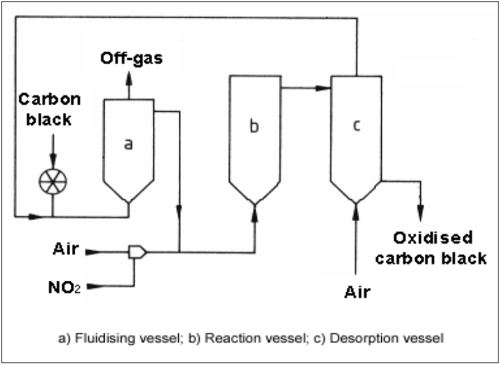
Surface oxidation of carbon black with nitric oxide and air can be carried out industrially in a fluidised bed reactor. A suitable post-treatment unit consists of a preheating vessel, in which the carbon black is fluidised and heated, a reaction vessel to carry out the surface oxidation, and a desorption vessel, in which adsorbed nitric oxide is removed.
Typical reaction temperatures lie between 200 and 300 °C. Depending on the degree of oxidation, the residence time can amount to several hours. The nitric oxide acts primarily as a catalyst, the oxygen in the air being the genuine oxidising agent. Oxidation of powdery black with ozone is also carried out on a commercial scale. Another common method of surface oxidation was carried out during pelletisation. Instead of water, nitric acid was used as the pelletising agent. The surface was oxidised while the wet beads were dried at an elevated temperature.
Source: European Commission, Large Volume Inorganic Chemicals - Solids and Others industry, August 2007, p. 215-216
Back to Subsection DG chemicals