Information about the chemical industry
Back to EFFICIENCY FINDER
1. GENERAL INFORMATION
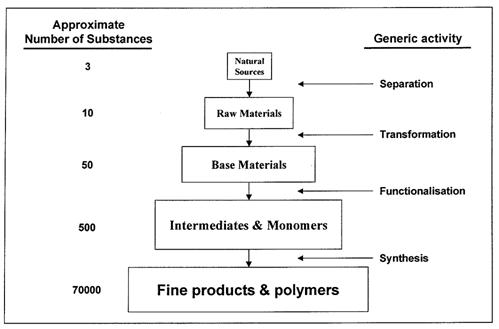
Industrial organic chemistry is characterized by the production of a huge variety of compounds in a step-wise manner from a few natural sources of carbon. This production pyramid is shown schematically in the following figure using typical chemical industry nomenclature:
Figure 1: Structure of Industrial Organic Industry, BREF in the Large Volume Organic Chemical Industry, February 2003
The initial separation steps are carried out in refineries where a few natural sources of carbon (crude oil, natural gas and coal) are used to produce a limited number of high volume raw materials for the chemical industry (e.g. naphtha). Some 95% of organic products are today obtained from oil and gas. Relatively few organic products come from the (declining) coal route and the (expanding) area of renewable biomass. Refineries export these raw materials to petrochemical plants where they are transformed by a complex combination of physical and chemical operations into a variety of base materials (e.g. ethylene, C3-C4 olefins, BTX aromatics, synthesis gas and acetylene). The base materials are subjected to further sequences of processing which introduce functional groups to produce an even greater number of intermediates and monomers (e.g. alcohols, aldehydes, ketones, acids, nitriles, amines, chlorides). The intermediates are converted into a large variety of fine products and polymers with high levels of functionalization and high commercial value (e.g. solvents, detergents, plastics, dyes and drugs). This production pyramid covers the whole spectrum of the organic chemical industry and the distinction between the tiers is often very subtle.
2. Organic Fine Chemicals
The chemical industry substantially contributes to the EU economy, with sales of over EUR 519000 million and a trade surplus of EUR 65000 million, making it Europe’s number one exporter. The chemical industry and all its sectors, especially the Organic Fine Chemicals (OFC) sector, is global in scope, competing on the world market with its products. The chemical industry is Europe’s third largest industry, employing 1.7 million people directly and with an additional 3 million jobs directly supporting the chemical industry. The OFC sector is estimated to employ over 0.6 million people with a turnover of EUR 125000 million. Typical employers include large multinationals with organic fine chemical business units, but over 90% of all sector companies are either middle-sized or SMEs.
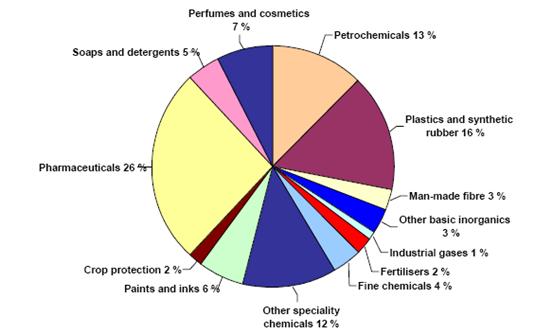
Figure 2: Sectoral breakdown of EU chemical industry sales (2003), BREF on Organic Fine Chemicals, Aug. 2006
Other speciality and fine chemicals are produced in smaller volumes than base chemicals. Speciality chemicals cover the auxiliaries for industry, dyes & pigments, oleo-chemicals, crop protection, and paints & inks. Fine chemicals represent pharma-intermediates, agrointermediates, and chemical intermediates. Pharmaceutical covers both basic pharmaceutical products and pharmaceutical preparations.
Organic fine chemical manufacturers produce a wide range of chemical substances, which are typically of a high added-value and produced in low volumes, mainly by batch processes in multipurpose plants. They are sold to companies, mostly other chemical companies, serving an immense range of end-user markets, on either a specification of purity or on their ability to deliver a particular effect. Typical major end-user markets are pharmaceuticals, agrochemicals, dyestuffs, flavours and fragrances, speciality polymers, electronics, food additives, and catalysts. Typical of the OFC sector is the manufacture of products or intermediates for internal use, in addition to external sales. The global market has grown at around 4 % per year and is currently valued at around EUR 265000 million (USD 300000 million).
OFC manufacturers range in size from very small (<10 staff) to very large multinationals (>20000 staff), with typical manufacturing sites having between 150 and 250 staff.
3. Polymers
- 3.1 Definition
Polymers – from Greek ‘poly’ (many) and ‘meros’ (parts) – are a group of chemical products which have a common building principle. They consist of so-called macromolecules which are long chain molecules, containing large numbers of smaller constitutional repeating units. Molecules consisting of a small number of monomers often are called ‘oligomers’ which means ‘some parts’. There are different types of polymers: natural polymers (for example wool, silk, wood, cotton), half synthetic polymers (natural polymers which are chemically modified, for example casein plastics, cellulose plastics) and synthetic polymers [27, TWGComments, 2004]. Monomers which mostly belong to the group of large volume organic products are nowadays usually produced from petrochemical feedstock (crude oil or gas). Exemptions are the cellulosic materials which are produced from cotton or wood fibres or biodegradable products produced from renewable raw materials.
- 3.2 Structure
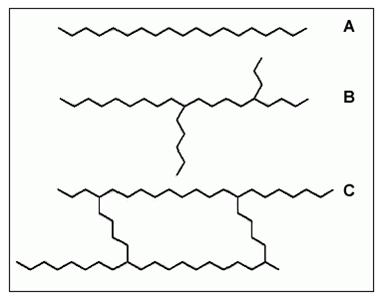
Macromolecules can be linear or branched (containing sidechains) and may be cross-linked, linking one chain with another. Examples of these three types of macromolecules are shown in Figure 3.
Figure 3: Basic structures of polymers
A) linear polymer
B) branched polymer
C) cross-linked polymer
Polymers can be composed from just one type of monomer (homopolymer) or from different types (copolymer). In the case of a linear copolymer consisting of two different monomers (e.g. A and B), the different monomers can basically be arranged in three different ways:
- random copolymer: there is no regularity in the arrangement of the two different monomers in the polymer
- block copolymer: blocks of pure A oligomer alternate with blocks of pure B oligomer
- alternating copolymer: the monomers A and B alternate within the composition of the polymer.
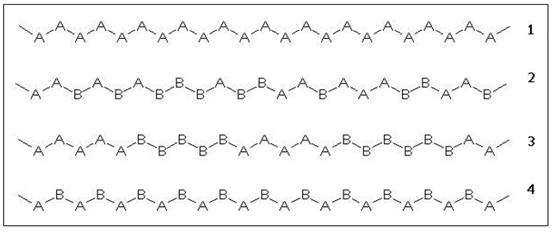
The composition and arrangement of the different monomers in a copolymer strongly influences its physico-chemical properties. Figure 4 shows the structure of a linear homopolymer and the three types of linear copolymer mentioned above.
Figure 4: Chemical composition of linear AB copolymers.
1) homopolymer
2) random copolymer
3) block copolymer
4) alternating copolymer
Apart from the linear copolymers, branched coploymers can be produced by grafting sidechains (consisting of monomer B) onto an existing homopolymeric mainchain (consisting of monomer A) (Figure 5).
Image:Composition of a graft copolymer.jpg
Figure 5: Composition of a graft copolymer
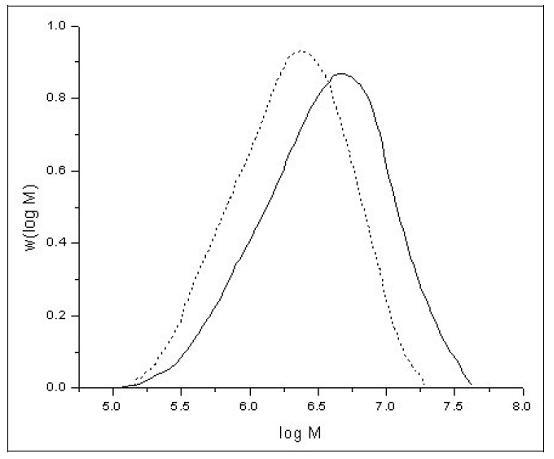
The polymerisation reactions are statistically driven processes. Therefore, unlike some natural polymers such as DNA, synthetic polymers always show, due to the reaction mechanisms involved in the production processes, a certain distribution of molar mass and not a distinct molecular weight. The molar mass of synthetic polymers can range from some thousand g/mol up to some million g/mol. As an example, Figure 6 shows the normalised molar mass distribution (MMD) curves of two different polyethylene samples.
Figure 6: Normalised molar mass distribution curves of two different polyethylene samples [29, M. Parth, et al., 2003]
Apart from molar mass and chemical composition, the properties of a polymeric material can be influenced by the shape of the MMD. The samples shown in Figure 6 both show a unimodal MMD, but to achieve some special mechanical properties, in some cases it is necessary to produce polymers with bimodal or multimodal MMD, as in natural polymers such as natural rubber (NR). This can be achieved by two subsequent polymerisation steps.
- 3.3 Properties
- 3.3.1 General properties
The underlying building principle is very flexible so that polymers with an extensive range of properties and property combinations can be produced. Polymers in the shape of objects, fibres or films may be:
- rigid or flexible
- transparent, translucent or opaque
- hard or soft
- weather resistant or degradable
- resistant to either high or low temperature.
In addition, they may be compounded with fillers, blended with other products (e.g. glass fibres) forming so-called composites or with other polymers yielding polymer blends. A certain polymer is usually not the only material which can be used in any given field of application. Alternative materials exist and polymers have to be successful in a competitive market. Polymers often bring advantages to numerous applications, for example:
- weight reductions and consequent transport and fuel savings
- electrical insulating properties suitable for wiring, switches, plugs, power tools and electronics
- optical transparency suitable for packaging, lighting and lens applications
- corrosion resistance which is important for plumbing, irrigation, rainwear and sports articles
- resistance to chemicals, fungi and mildew
- ease of processing making complicated shapes possible
- cost savings over alternative solutions.
- 3.3.2 Thermal properties
Usually, substances can exist in three possible physical states: solid, liquid and gas. In polymeric materials, things are not so straightforward. For example, most polymers will decompose before they boil, and cross-linked polymers decompose before they melt. According to their basic thermal properties, four different types of polymers are distinguished.
- Thermoplastics
- Thermoplastics are polymeric materials, which are more or less rigid at room temperature and can be melted by heat.
- Thermosets
- Thermosets are also rigid at room temperature, but due to the cross-links in their molecular structure, they cannot be melted.
- Rubbers or elastomers
- Rubbers are flexible at room temperature. Most of them are amorphous materials and do not show a melting point. They have a glass transition point instead which is well below room temperature. Below this glass transition temperature they are rigid.
- Thermoplastic elastomers
- Thermoplastic elastomers are block copolymers or polymer blends that are flexible and show properties similar to vulcanised rubbers at room temperature, but which can be softened or melted by heat. This process is reversible, so the products can be reprocessed and remoulded.
- 3.4 Main uses
- 3.4.1 Fields of application
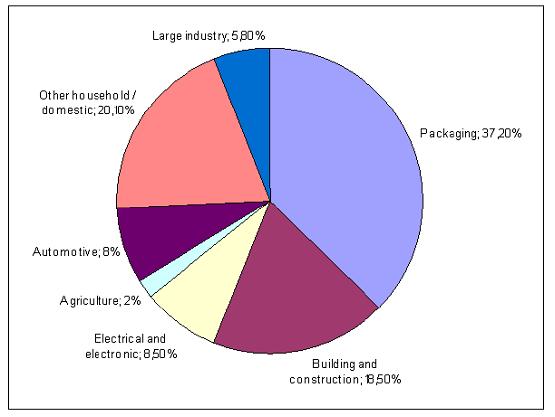
Polymeric materials are used in simple household items like plastic bags as well as in advanced optical or electronic components or in medical applications. The main fields of application for Western Europe are shown in Figure 7. which does not include data about elastomers and cellulosic fibres. For 2003, the total amount of consumed thermoplastics and thermosets in Western Europe was 48788 kilotonnes.
Figure 7: Main uses for polymers in 2003
- 3.4.2 Processing technologies
A range of processing technologies are used to convert raw polymers into the required shape of the final product. This conversion step is normally entirely separate from the manufacturing site of polymer pellets. The processing step itself is mainly a physical transformation step using different technologies such as:
- extrusion for pipes, profiles, sheets and cable insulation
- injection moulding for products of different, often very complex shapes like machine parts, electrical plugs and medical equipment such as syringes; thermoplastics and thermosets
- blow moulding for bottles, containers and films
- calendering for films and sheeting
- rotomoulding for large shapes
- pultrusion for rods, tubes, etc.
- blown film for thermoplastics
- cast film for thermoplastics
- coating for thin layers on different substrates
- pressing for resins
- spinning for fibres
- transfer moulding for thermosets
- compression moulding for thermosets
- vulcanisation for rubbers
- blending generally applicable technique.
Usually, chemical reactions do not occur during these processing steps, except during the vulcanisation of rubber, during the in-process cross-linking of certain types of cable insulations made from polyethylene and when processing certain resins with in-situ polymerisations. Such special processing steps are described in literature [14, Winnacker-Kuechler, 1982].
- 3.5 Main products
- 3.5.1 Polymers based on crude oil
Different market requirements have resulted in a wide range of polymeric materials which are grouped into:
structural materials where the polymer is the main and most visible structural component with the subgroups:
- commodity polymers (polyethylene, polypropylene, polystyrene, polyvinyl chloride, ESBR, etc.)
- Such polymers are used in large quantities at relatively low costs for major applications like tubes, films, profiles, containers, bottles, sheets, tyres, etc.
- engineering polymers and speciality rubbers (ABS, polyamides, polyesters, polyacetals, polymethyl methacrylates, EPDM, NBR, etc.)
- Such polymers are used for special requirements at an intermediate cost level often for very small parts (clips, valves, special machine parts, etc.)
- high performance products (polyimide, polytetrafluoroethylene, polysulfone, polyetherketone, fluorinated and silicone rubbers, etc.)
- Such low volume, high priced materials are used to meet extreme requirements like high temperature, weather or solvent resistance, special wear or optical properties, extreme purity for critical medical applications, etc.)
- thermosetting polymers (polyesters, epoxies, phenolics and alkyd resins)
- They are often used as coating resins and binders for fibrous reinforcements in a range of applications from boats to brake linings and functional materials where polymers are used as an aid to achieve a special function. They mostly constitute a small and often invisible part of the total system only with the following subgroups:
- commodity applications like dispersants, detergents, flocculants, thickeners, superabsorbers or adhesives and glues
- Here, large volume polymers based on polyvinyl acetate, polyacrylic acid and its derivatives, and polyvinyl alcohol are used
- special technical applications like membranes, optical fibres, products with electrical conductivity, and light emitting products
- Here, high priced materials are used in small amounts where the functionality, and not predominantly the mechanical properties, is important.
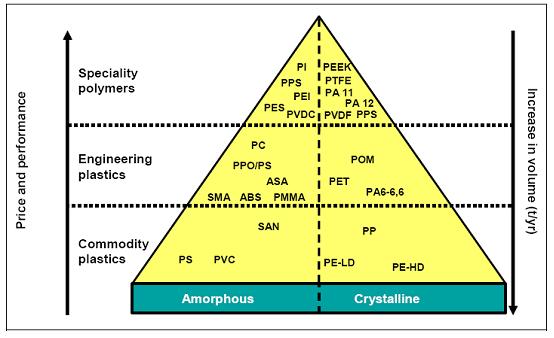
A classification of thermoplastic products (not including elastomers and thermosetting resins) is shown in Figure 8.
Figure 8: Classification of thermoplastic polymers
Generally, amorphous polymers have a disordered structure, have a softening point and are very often transparent, while crystalline polymers have an ordered structure, have a softening and a melting point and are mostly opaque. Amongst the polymers based on crude oil, seven groups of polymers – polyolefins (PE and PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), emulsion polymerised styrene butadiene rubber (ESBR), polyamides (PA) and unsaturated polyester resins (UP) constitute approximately 80 % of the total consumption of polymers. Within each product group, there exists a wide variety of individual product grades optimised for the specific application (tailor-made).
For example:
- PE with good flow properties for injection moulding or, for instance, boxes or containers
- PE with excellent long-term stability for pipes
- PE with good blow moulding properties for petrol tanks in automobiles.
They are not interchangeable for these specific applications. Some have a low molecular weight; some have a high molecular weight, and while some have a narrow molecular weight distribution, others offer an extremely wide molecular weight distribution. The final mechanical, rheological and other physical properties depend on these parameters.
- 3.5.2 Polymers based on renewable resources
Historically, the first polymers were produced from renewable resources:
- fibres from cellulose (cotton) or derivatives (cellulose acetate)
- fibres from polypeptides (wool)
- plastics from cellulose acetate
- rubber from tree resin (polyisoprene).
While some of these products stayed competitive (rubbers, viscose fibres), others – especially in the field of thermoplastic material applications – did not, mainly for economic reasons or insufficient properties but sometimes also due to high environmental costs. Newer attempts to develop wood-based plastics (‘synthetic wood’) remained limited to niche applications (laminates for flooring, boats, musical instruments). Corn derived products (e.g. polylactic acid) and blend systems of starch and petrochemically produced polymers present new opportunities to use renewable resources as raw materials for plastics. Generally, renewable raw materials can be used to produce either long-term living products like construction materials for automobiles, ships and for the building and construction sector, or short-term living products like compostable packaging or biodegradable mulch films.
- 3.5.3 Biodegradable polymers
The market for biodegradable materials is limited to niche applications. General politically motivated goals in the past, like substituting commodity products for environmental reasons, provoked several costly industrial developments over many years. Finally, some of them proved unrealistic since the alternatives failed in properties as well as in processability and economics and sometimes also due to an undefined environmental outcome. This class of polymers is not described in this document because their production in the European Union currently does not represent a significant environmental impact. Today, biodegradable products are developed for markets where biodegradability is considered a technical advantage like for instance:
- mulch film in agriculture
- garbage bags for composting which can provide easier handling and eco-efficient benefits for waste management
- paper coating
- hygiene films including funeral applications, sanitary towels.
Biodegradability does not depend on the origin of the raw materials but on the chemical structure. Thus, materials from renewable as well as from synthetic resources are on the market. While cellophane, starch and polyhydroxybutyrate have existed on the market for many years, newer developments include poly (L-lactide) as well as numerous fossil based biodegradable polymers, e.g. copolyesters. A legal situation recognising organised composting as one means of recycling and a standardised testing of the degradation behaviour are important preconditions for their successful development. The total market segment requiring biodegradability is currently estimated to be about 50 - 200 kt/yr in Western Europe. The actual consumption is around 8 kt/yr according to CEH Marketing Research Report in the Chemical Economics Handbook – SRI International 2000.
- 3.6 Processes and techniques applied in the production of polymers
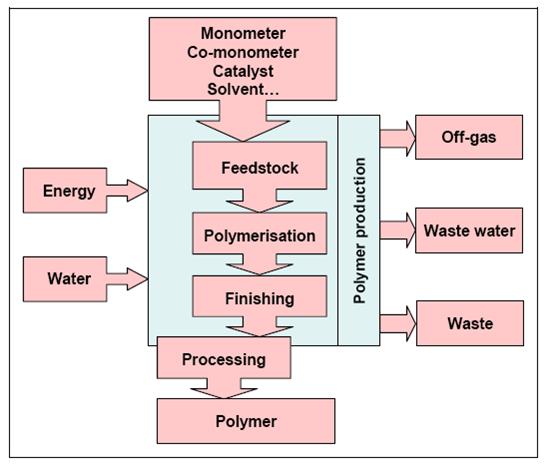
The production of polymers follows the scheme given in Figure 9 with monomers, comonomers, catalysts, solvents as well as energy and water on the input side and the product, off-gases, waste water and wastes on the output side.
Figure 9: General production scheme
- 3.6.1 Energy
Energy is needed for the production of polymers, even in the case of polymerisation systems where the process itself is exothermic, i.e. generates energy. The demand for energy also depends on the local situation if the polymerisation unit is integrated into a larger complex with, for example, the need for low pressure steam or not. Thus, the swap of energy between different plant sites has to be taken into account.
- 3.6.2 Chemical reactions
The production of polymers consists essentially of three parts:
- * preparation
- * reaction step
- * separation of products.
- Polymerisation (chain growth reaction)
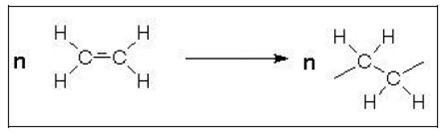
- Polymerisation is the most important reaction process and produces amongst others the plastics polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and polystyrene (PS). The reaction principle includes the opening of the double bond of a monomer (Figure 10) and linking many monomeric molecules together forming a saturated long chain macromolecule. These reactions are usually exothermic, thus producing energy.
Figure 10: Polymerisation by the opening of a double bond (e.g. ethylene)
- The number of molecules combined, n, may vary at the low end between 10 – 20. The products are then called telomers or oligomers. For polymers, n is between 1000 and 100000 or more. The polymer growth occurs very rapidly, in seconds or minutes. Thus, fully formed macromolecules exist almost from the beginning of the reaction. However, the overall time required for a high conversion of monomer to polymer is often several hours.
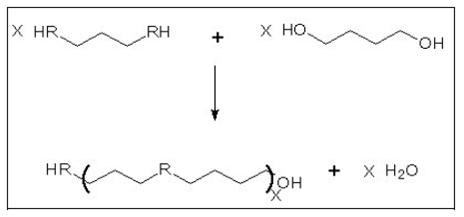
- Polycondensation (step growth reaction)
- The reaction principle includes the reaction of a monomer with two distinctive reactive functional groups or the combining of two bifunctional monomers forming a polymer and generating a by-product which is, in many cases, water. A schematic view of the reaction is shown in Figure 11.
Figure 11: Schematic view of a polycondensation reaction
- The reactive groups may be for instance:
- alcohol plus acid for polyesters
- amine plus acid for polyamides.
- This process is, like most of the chemical reactions, an equilibrium process; it may be shifted in either direction depending on the conditions. High yields are achieved only by careful removal of the by-products (water or alcohols) which are formed. Otherwise, the by-product would interfere and reduce the molecular chain length. The by-product is removed by heat and by high vacuum towards the end of the reaction. This gets increasingly problematic as the viscosity of the reaction medium increases. Sometimes, a thermal after-treatment in the solid phase is used to increase the molecular weight even further. In any case, a special reactor design is needed for the last phase of the reaction.
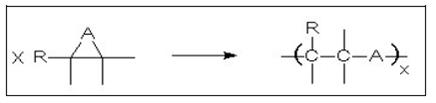
- Polyaddition
- The reaction principle includes the opening of a reactive ring, or a reactive group forming a polymer (see Figure 12).
Figure 12: Schematic view of a polyaddition reaction
- If A is an oxygen atom, polyepoxides are obtained; if the ring reacts with another bifunctional group like diols, diamines or carbonic acid anhydrides, epoxy resins are formed.
4: BIBLIOGRAPHY
- 1. BAT in the Large Volume Organic Chemical Industry, February 2003
- 2. BAT for the Manufacture of Organic Fine Chemicals, August 2006
- 3. BAT for Polymers, October 2006
Back to EFFICIENCY FINDER