Optical brighteners
Back to Information about optival brighteners
1 Overview
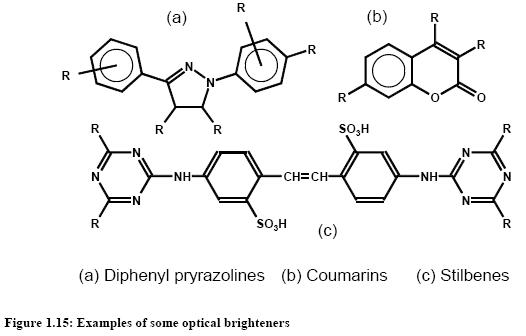
Optical brighteners or, more accurately, fluorescent whitening agents, are colourless to weakly coloured organic compounds that, in solution or applied to a substrate, absorb ultraviolet light (e.g. from daylight at c. 300 – 430 nm) and re-emit most of the absorbed energy as blue fluorescent light between c. 400 and 500 nm. In daylight, optical brighteners can thus compensate for the aesthetically undesirable yellowish cast found in white industrial substrates, such as textiles, papers, or plastics. Figure 1.15 gives some examples.
2 Production of Optical Brighteners
- Reduction of aromatic nitro compounds
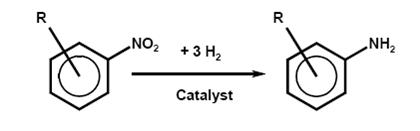
One of the most industrially important reduction processes in industrial use is the conversion of an aromatic nitro or dinitro compound into an arylamine or arylene diamine. Aromatic amines are widely used as dye intermediates, especially for azo dyes, pigments, and optical brighteners; as intermediates for photographic chemicals, pharmaceuticals, and agricultural chemicals; in polymers via isocyanates for polyurethanes; and as antioxidants.
Among reduction methods, there are three of major relevance in organic fine chemistry:
- catalytic hydrogenation, which is extremely important industrially because of its universal applicability; most processes can be carried out successfully by catalytic hydrogenation
- Béchamp and Brinmeyr reduction with iron, which is the classical method
- alkali sulphide reduction, which is the selective method in specific cases, such as in the manufacture of nitroamines from dinitro compounds, the reduction of nitrophenols, the reduction of nitroanthraquinones and the manufacture of aminoazo compounds from the corresponding nitroazo derivative.
All three methods are also applied to halogenated nitro compounds, and can thus contribute to AOX loads in waste water streams.
- a) Catalytic reduction with hydrogen
Chemical reaction
The catalytic reduction of the nitro compounds is very exothermic. To reduce these hazards, the concentration of nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst, are controlled.
Most aromatic nitro compounds are hydrogenated in the liquid phase. In this case, the pressure and temperature can be changed independently. The temperature is limited by the hydrogenation reaction of the aromatic ring which occurs above 170–200°C.
Normally, the reduction is carried out at 100–170°C. Sensitive compounds are hydrogenated at lower temperatures (20–70°C) or at lower pressures (1–50 bar). 1–50 bar are used normally.
The preferred solvents are methanol and 2-propanol; and also dioxane, tetrahydrofuran, and N-methylpyrrolidone are used. In the hydrogenation with a water immiscible solvent, such as toluene, the water must be removed, as in solvent-free hydrogenation, in order to maintain the activity of the catalyst. If the amine has a good water solubility, water is used as the solvent. Water also can be used in cases where the nitro compound forms water-soluble salts with alkalis, such as with nitrocarbonic or sulphonic acids. In practice, only Raney nickel, Raney nickel-iron, Raney cobalt, and Raney copper are used as pure metal catalysts because of their relatively low cost. Precious metal catalysts, such as Pt and Pd, are generally used at concentrations of 0.5 – 5 wt-% on support material with large surfaces, such as charcoal, silica, aluminium oxide, or alkaline-earth carbonates.
Process hazards
The catalytic reduction of nitro compounds is very exothermic. Unless this heat is dissipated properly, decomposition and even explosions can result, especially if the thermal decomposition of the nitro compound occurs or if condensation reactions are initiated as may be the case with chloro-nitro compounds. The industrial hydrogenation of aromatic polynitro compounds in the liquid phase without solvents especially requires precautions. To reduce these hazards, the concentration of the nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst are controlled. The nitro compound is continuously added in small quantities, thus keeping its concentration below 2 %. De-ionised water is added to remove the heat of the reaction by continuous evaporation and to slow down the activity of the catalyst.
Operations
The vast majority of aromatic amines have small annual volumes (<500 tonnes) and are produced by batch hydrogenation with catalyst slurries. The reaction is carried out in stirred, steel or stainless steel autoclaves or in loop reactors. Loop reactors show increased heat and mass transfers and improved reaction selectivity, shorter batch cycle times and higher product yields. In addition, catalyst usage is often lower. The addition sequence depends on the particular reactants. On completion the reaction mass is cooled and the catalyst is removed by filtration.
- b) Reduction with iron
Chemical reaction
The reduction of nitroaromatics is carried out in the presence of small amounts of acid (HCl, H2SO4, HCOOH, CH3COOH) as shown in the following equation:
4 Ar – NO2 + 9 Fe + 4 H2O 4 Ar – NH2 + 3 Fe3O4
The acid is used for the activation of the iron. Only 2 – 3 % of the hydrogen is derived from the acid but 97 – 98 % comes from the water.
Operations
Normally the nitroaromatic is added to the mixture of iron/water/acid (excess of iron about 15-50 %) often in the presence of an organic solvent (toluene, xylol, alcohols) and the mixture is heated to reflux. Depending on the reactivity of the aromatic, other addition sequences may be required. In some cases the acid is omitted (neutral iron reduction). The build-up of unreduced excess nitro compound must be avoided and the final mixture should be tested for its total absence. After basification with soda ash (anhydrous sodium carbonate) to precipitate soluble iron, the iron compounds are removed by filtration.
- c) Alkali sulphide reduction
Chemical reaction
The alkali sulphide reduction is a mild and selective reaction according to the following equation, without strict stoichiometry:
Ar – NO2 + Na2S2 + H2O Ar – NH2 + Na2S2O3
Other reducing agents in use are Na2S or NaSH, which also form Na2S2O3. Sulphur may be added to reduce the required amount of sulphide.
Operations
Dilute aqueous sulphide is added to the solution or emulsion of the nitro compound. Temperatures (in the range of 80–100°C) and concentrations depend on the reactivity of the nitroaromatic. An excess of sulphide is avoided in the case of the selective reduction of polynitro compounds.
PRODUCT WORK-UP:
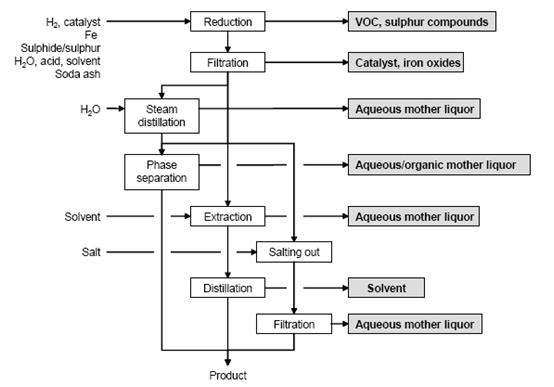
Figure 1 shows a typical sequence of operations for the reduction of aromatic nitro compounds, possible input materials and associated waste streams. The work-up depends on the properties of the amine obtained. Common methods are:
- separation as a liquid
- cooling and salting out
- steam distillation
- extraction with organic solvent, and
- pH adjustment if necessary.
Figure 1: Typical sequence of operations for the reduction of an aromatic nitro compound. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
Literature: BAT for the Manufacture of Organic Fine Chemicals, August 2006