Bleaching in textile industry
Back to EFFICIENCY FINDER
Back to EFFICIENCY FINDER FOR TEXTILE INDUSTRY
1. OBJECTIVE
Bleaching is applied to remove pigments and natural dyes that are in the fibres and give a sort of coloration. When the material has to be dyed in dark colours it can be directly dyed without need of bleaching (BAT for the Textiles Industry, July 2003).
2. FIELD OF APPLICATION
Bleaching can be performed on all kinds of make-ups (BAT for the Textiles Industry, July 2003):
- yarn
- woven
- knitted fabric
3. DESCRIPTION OF TECHNIQUES, METHODS AND EQUIPMENT
The most frequently used for cellulosic fibres are oxidative bleaches, namely (BAT for the Textiles Industry, July 2003):
1) hydrogen peroxide (H2O2)
2) sodium hypochlorite (NaClO)
3) sodium chlorite (NaClO2).
1) Bleaching with hydrogen peroxide
Bleaching can be carried out as a single treatment or in combination with other treatments (e.g. bleaching/scouring or bleaching/scouring/desizing can be carried out as single operations). The textile is treated in a solution containing hydrogen peroxide, caustic soda and hydrogen peroxide stabilisers at pH 10.5 – 12. Other auxiliaries used in hydrogen peroxide bleaching are surfactants with emulsifying, dispersing and wetting properties. Employed surfactants are usually mixtures of anionic compounds (alkyl sulphonates and alkyl aryl sulphonates) with non-ionic compounds such as alkylphenol ethoxylates or the biologically degradable fatty alcohol ethoxylates.
Operating temperatures can vary over a wide range from ambient to high temperature. Nonetheless, a good bleaching action occurs when operating at around 60 – 90 ºC. Bleaching with hydrogen peroxide in neutral conditions (pH range of 6.5 – 8) is also possible in some cases (e.g. when treating cotton in blends with alkali-sensitive fibres such as wool). At these pH conditions activators are required to give bleaching activity. Note that below pH 6.5 H2O2 decomposes into H2O and O2 by HOO * - / O2 * disproportionation. Under these conditions hydrogen peroxide is wasted (production of inactive O2 gas).
A wide range of bleaching processes can be used, including cold pad-batch, bleaching under steaming conditions and bleaching processes in long bath.
Because the bleaching agent of peroxide is anionic in nature (hydrophilic behaviour), it is not possible with this bleaching method to destroy selectively the coloured hydrophobic material present on natural fibres without attacking the polymer itself.
2) Bleaching with sodium hypochlorite
The high reactivity of this bleaching agent imposes softer operative conditions than hydrogen peroxide (pH 9 - 11 and temperatures not above 30 ºC). Otherwise there is a risk of damage to the cellulose fibre.
The bleaching stage is followed by an anti-chlorine treatment in order to eliminate completely the hypochlorite and decompose the chloroamines generated during bleaching.
Bleaching with sodium hypochlorite can be carried out in batch (e.g. overflow, jet, jigger, winch beck), semi-continuous (pad-batch) or continuous mode. A two-stage process is also in use in which hypochlorite and hydrogen peroxide are used.
The use of hypochlorite as bleaching agent is in decline for ecological reasons. It is still applied for yarn and knitted fabric when a high degree of whiteness is required, for articles that remain white (e.g. linen), or require a white background or in processes where the ground-dye is discharged with a bleach treatment.
3) Bleaching with sodium chlorite/ chlorate
Chlorite/chlorate bleaching, although in decline, is still applied for synthetic fibres, cotton, flax and other cellulosic fibres, often in combination with hydrogen peroxide.
The bleaching agent is the chlorine dioxide gas (ClO2), which follows a completely different working mechanism compared to hydrogen peroxide. Whereas the superoxide radical ion in hydrogen peroxide is hydrophilic and therefore works preferentially in the hydrophilic region of the fibre (attack of the fibre polymer), ClO2 absorbs preferentially on the hydrophobic associated material, such as the woody part of bast fibres. For this reason it is an excellent bleaching agent (ensuring a high degree of whiteness and no risk of damage of the fibre) especially for synthetic fibres and for bast fibres such as flax where, compared to cotton, there is a higher percentage of hydrophobic impurities.
Because chlorine dioxide is unstable as a gas and can only be stored as a solution of approximately 1 % in water, it must be generated on-site as an aqueous solution. There are two ClO2 precursor chemicals in present general industrial use, namely sodium chlorite and sodium chlorate. Although sodium chlorate is considerably less expensive than sodium chlorite, it is more difficult and expensive to convert to ClO2, which explains why it is less commonly used.
Both sodium chlorite and sodium chlorate are used in strong acid conditions (pH 3.5 – 4 by formic or acetic acid). Chlorine dioxide solutions have a great corrosive action on construction materials including stainless steel. Sodium nitrate is used as a corrosion inhibitor to protect the stainless steel parts of equipment. It is also necessary to select detergent/wetting agents that can resist acid conditions. On the other hand, sequestering agents are not necessary because the oxalic acid used for acidification also serves for sequestering metals. The order of introduction of the different auxiliaries has to be controlled to avoid direct contact between the concentrated sodium chlorite/ chlorate solution and acids.
The textile material is bleached by padding or in long bath processes. The temperature is normally kept at 95 ºC, but cold procedures have also been developed to diminish toxicity and corrosion problems, using formaldehyde as an activator for sodium chlorite.
The advantages of chlorine dioxide bleaching are the high degree of whiteness and the fact that there is no risk of damage to the fibre. The main disadvantages are the high stresses to which the equipment is subjected and the chlorine residues that may be left on the fibre, depending on the way chlorite (or chlorate) is produced and activated. Recent technologies using hydrogen peroxide as the reducing agent of sodium chlorate are now available to produce ClO2 without generation of AOX.
4) Bleaching with peracetic acid
Peracetic acid is produced from acetic acid and hydrogen peroxide. It can be purchased as ready-made product or produced in-situ. Its optimal bleaching action is reached only in a very narrow pH range between 7 and 8. Below pH 7 the degree of whiteness decreases sharply and above pH 9 depolymerisation of the fibre with consequent damage of the fibre occurs.
Peracetic acid is sometimes applied for synthetic fibres (e.g. polyamide) where hydrogen peroxide cannot be used.
4. COMPETITIVE TECHNOLOGIES AND ENERGY SAVING POTENTIALS
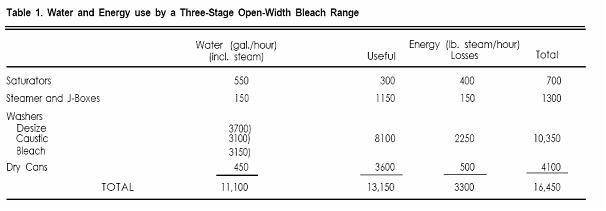
There is a strong correlation between water and energy use in textile bleaching, since a high proportion of the energy is used for heating wash water. Thus by reducing the water consumption of a bleach range significant savings in energy can also be realized. A typical open-width bleach range consists of desize, caustic, and peroxide bleach stages. Each stage has a washing train, which requires a major water feed, usually 50-75 gal./min., which is counterflowed through all the washers in that stage. (Data used in this discussion are based on 60 gal./min. at the desize washers and 50 gal/min. each at the caustic and bleach washers.) Water temperatures vary but are almost always at least 180°F. The water and energy use for such a bleach range are shown in Table 1. Of the total 16,450 lb. steam/hour required to operate the bleach range, 10,350 are used by the washing systems--this figure includes 8100 lb. steam/hr. needed to achieve 180oF and 2250 lb. steam/hr. to compensate for heat losses. Energy losses are mainly evaporative and could be reduced substantially if enclosed washers were used. There is an obvious opportunity for saving both water and energy by reducing water flows and temperatures on the bleach range.
(“Potential water and energy savings in textile bleaching”, by Bruce A. Evans, E. I. DuPont deNemours & Co., Inc., Chemicals and Pigments Department, Chestnut Run, Wilmington, DL 79898, http://www.p2pays.org/ref/03/02903.pdf#search=%22Bleaching%20textile%20energy%20savings%22)
We will discuss the following options:
- 1. Reduce water temperature
- 2. Reduce water flow rates
- 3. Reduce the number of water feeds
- a. by counterflowing between stages
- b. by reducing the number of stages
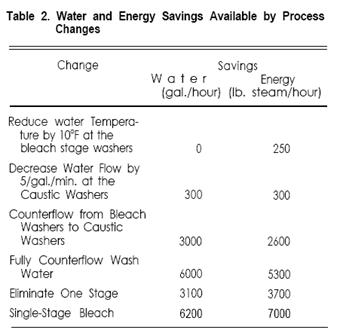
The estimated savings available by making these changes are summarized in Table 2.
1. Reduce water temperature
The bleach washers remove a relatively small share of the impurities in the fabric, and reductions in water temperature are best considered there. As shown in Table 2, a decrease of 10°F from 180°F to 170°F at the bleach washers would decrease energy requirements by 250 lb./steam/hour; it would not reduce the water requirement at all. For most bleach ranges this is a relatively safe change. In fact many mills run 150°- 160°F water in the bleach washers.
2. Reduce water flow rates
For the desize and caustic stages, temperature reductions are not generally advisable because the materials being removed (caustic, sizing polymers, waxes) become more viscous or fall below their melting points in cooler water.
However, a reduction in the water flow rate of 5 gal./min. would save 300 gal./hour water and 300 lb. steam/hour energy. This is attractive if it can be reliably determined that more water than necessary is being used to remove the impurities. There are some potential problems, though, which make it difficult to lower flow rates: + Many mills do not control flow rates very well, and would not recognize a reduction if it occurred. The bleach range operator often controls the flow valve at his own whim. Flow rates should not be lowered until they are controlled and until fabric quality has been monitored with the present rates. + No automatic monitors are available to measure the concentration of impurities in the wash water or on the fabric. Data on the effects of changes must be gathered by painstaking laboratory work. + In the absence of other controls, the water flow is set to be sufficient for the most difficult fabrics processed. It is generally changed for more easily washed fabrics. (A control was recently introduced which allows automatic variation of wash water flow with the lb./hour of fabric produced-this assumes that the difficulty of washing is proportional to fabric weight, which is reasonable for most types of fabric.) One valid way of reducing water flow is to use more efficient washers. Horizontal washers use about one third to one-quarter as much water as vertical washers for the same washing job. Water and energy use is reduced accordingly.
3. Reduce the number of water feeds
- a) Counterflow between Stages:
As stated earlier the standard open-width continuous bleach range has three separate water feeds. Counterflowing water from the bleach washers to the caustic washers could eliminate 3000 gal./hour water feed and could save 2600 lb. steam/hour. A mass-energy balance of a typical bleach range has shown that the total impurities in the bleach wash water as it is discharged are about 0.03 lb./gallon, or 0.34% by weight. It is clean enough for re-use in the desize or caustic stage for most mills, and many of them have recently begun to take advantage of it. It is possible to recycle the same water through all three stages, but it is rarely done. Wash water used in both the bleach and caustic stages will contain about 1.3% impurities by weight, and its re-use in the desize stage might lower washing efficiency too much. There is also the possibility of coagulation of impurities due to cooling during the counterflowing. In spite of the high incentive (6000 gal./hour and 5300 lb. steam/hour) full counterflow is practiced on very few ranges.
- b) Reduce the number of stages:
Elimination of a bleach range stage means elimination of a saturator, steamer and water feed, which saves 3100 gal./hour and 3700 lb. steam/hour. There are three options: + Combining desize and caustic functions. This option is especially attractive for polyester/cotton blends, because polyvinyl alcohol is often used as size and because caustic levels are kept low to protect the polyester. Since polyvinyl alcohol is removed by hot water washing, the system is really a “NO-DESIZE” one. A small addition of hydrogen peroxide, stabilized with Epsom Salt, can assist in removal of the polyvinyl alcohol and other minor sizing polymers which may be present. + Combining caustic and bleach functions. If the caustic stage is eliminated the result is a desize-bleach system; DuPont introduced this concept during the 1950’s as the Solo-Matic process. The most popular configuration is rope desizing in bins followed by a single J-Box to perform the functions of both caustic and bleach stages. It is intended primarily for relatively light-weight open-weave fabrics including soft-filled sheeting, polyester/cotton shirtings, and prepares for printing and non-critical dyeing. Its major limitations are development of absorbency and removal of motes, both of which require a strong caustic treatment. The bleach bath in a desize-bleach system uses high alkali content and requires sodium silicate buffering to pH 10.8-11.0; 1.25-1.50 hours of steaming time are needed to produce good results. Because of the long steaming time there is no comparable open-width process available. + Single Stage Bleaching. The prospect of eliminating two stages, including saturators, steamers, and washers has resulted in widespread interest in single-stage bleaching. Present rope applications include knit goods, bedsheets, terry cloth, and light weight polyester/cotton shirtings. There are very few open-width single-stage bleach ranges because of the limited steaming times available; a few plants run corduroy and other fabrics for non-critical dyeing use on a single-stage ranges, but results are often marginal. The conveyor steamer, with 15-20 minutes steaming time, is of greatest interest for open-width single-stage bleaching, although few such applications are presently running.
4. Pre-washing:
In any single-stage system and in many other abbreviated bleach configurations it will be beneficial to use a prewashing train with recycled water from the bleach stage--this might be called a l-l/2 stage range. Prewashing can remove significant quantities of sizing and natural impurities which would otherwise have to be removed by the final washers, and which might affect the stability of the bleach bath. Since prewashing with recycled water does not increase water consumption and has very little impact on energy use, it is always recommended.
5. Conclusion
There are significant potential savings in both water and energy available by carefully selecting the optimum bleach range configuration for the types of fabrics being processed. In some textile areas the cost of water including waste treatment exceeds $1.00/1000 gallons. Energy as oil-fired steam costs about $7.00/1000 lb. delivered to the bleach range. At these costs escalate, the potential savings will grow proportionately. As an example, using the above costs, the process change “Counterflow from Bleach Washers to Caustic Washers” (Table 2) would save about $20,000/year water cost and $ll0,000/year energy cost, for a total saving of $130,000/year. Realization of such savings requires careful optimization of the bleach range configuration for the types of fabrics being processed, constant control of water and energy use, and careful attention to equipment maintenance to avoid waste. Textile finishing plants generally recognize the opportunity and necessity of saving water and energy and are making rapid progress in that endeavour.
Back to EFFICIENCY FINDER