Dyeing in textile industry
Back to EFFICIENCY FINDER FOR TEXTILE INDUSTRY
1. OBJECTIVE
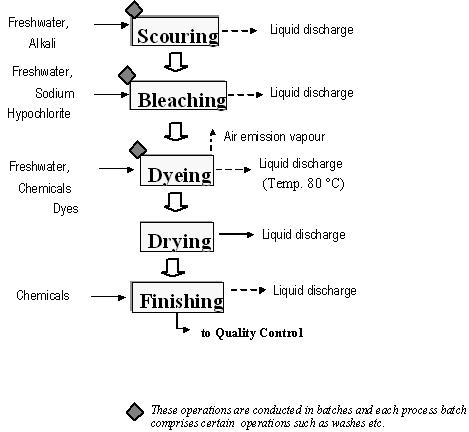
Dyeing is a method for colouring a textile material in which a dye is applied to the substrate in a uniform manner to obtain an even shade with a performance and fastness appropriate to its final use. A dyestuff is a molecule which contains a chromophoric group (conjugated system) capable of interacting with light, thus giving the impression of colour (BAT for the Textiles Industry, July 2003).
2. FIELD OF APPLICATION
Textiles can be coloured at any of several stages of the manufacturing process so that the following colouring processes are possible (BAT for the Textiles Industry, July 2003):
- flock or stock dyeing
- top dyeing: fibres are shaped in lightly twisted roving before dyeing
- tow dyeing: it consists in dyeing the mono-filament material (called tow) produced during
- the manufacture of synthetic fibres
- yarn dyeing
- piece (e.g. woven, knitted and tufted cloths) dyeing
- ready-made goods (finished garments, carpet rugs, bathroom-sets, etc.).
3. DESCRIPTION OF TECHNIQUES, METHODS AND EQUIPMENT
Various dyeing techniques exist (BAT for the Textiles Industry, July 2003):
- mass dyeing/gel dyeing, in which a dye is incorporated in the synthetic fibre during its production (this technique is the most commonly applied process for PP fibres and also for PAC;
- pigment dyeing, in which an insoluble pigment, without affinity for the fibre, is deposited onto the textile substrate and then fixed with a binder;
- dyeing processes which involve the diffusion of a dissolved or at least partially dissolved dye into the fibre.
Dyeing can be carried out in batch or in continuous/semi-continuous mode. The choice between the two processes depends on the type of make-up, the chosen class of dye, the equipment available and the cost involved. Both continuous and discontinuous dyeing involve the following steps (BAT for the Textiles Industry, July 2003):
- preparation of the dye
- dyeing
- fixation
- washing and drying.
- Batch- dyeing: (BAT for the Textiles Industry, July 2003)
In batch dyeing (also called exhaustion dyeing) a certain amount of textile material is loaded into a dyeing machine and brought to equilibrium with a solution containing the dye and the auxiliaries over a period of minutes to hours.
The dyeing process starts with the absorption of the colourant onto the external surface of the fibre, then the diffusion and migration of the colourant through the fibre takes place. The use of chemicals and controlled temperatures accelerates and optimises exhaustion and fixation (rate/level) of the dye. When the dyeing is judged to be the right shade, the spent dye bath is drained and the textile material is washed to remove unfixed dyes and chemicals. Washing is usually carried out in the same equipment. However, separate washing machines can also be used in the case of fabric.
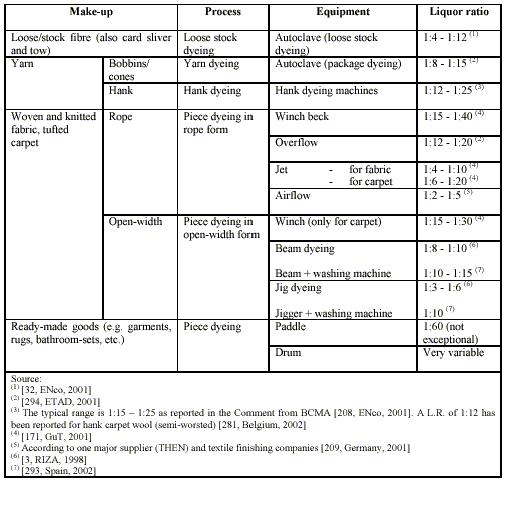
An important parameter in discontinuous dyeing is the liquor ratio of the equipment. This is the weight ratio between the total dry material and the total liquor. So, for example, a liquor ratio of 1:10 means 10 litres of water on 1 kg textile material. This parameter influences the amount of water and energy consumed in the dyeing process. Dyeing machines vary greatly in their liquor ratios, depending also on the type of substrate to be dyed and its hydrophilicity.
Table 1 shows typical ranges of Batch- dyeing: (BAT for the Textiles Industry, July 2003)
In batch dyeing (also called exhaustion dyeing) a certain amount of textile material is loaded into a dyeing machine and brought to equilibrium with a solution containing the dye and the auxiliaries over a period of minutes to hours.
The dyeing process starts with the absorption of the colourant onto the external surface of the fibre, then the diffusion and migration of the colourant through the fibre takes place. The use of chemicals and controlled temperatures accelerates and optimises exhaustion and fixation (rate/level) of the dye. When the dyeing is judged to be the right shade, the spent dye bath is drained and the textile material is washed to remove unfixed dyes and chemicals. Washing is usually carried out in the same equipment. However, separate washing machines can also be used in the case of fabric.
An important parameter in discontinuous dyeing is the liquor ratio of the equipment. This is the weight ratio between the total dry material and the total liquor. So, for example, a liquor ratio of 1:10 means 10 litres of water on 1 kg textile material. This parameter influences the amount of water and energy consumed in the dyeing process. Dyeing machines vary greatly in their liquor ratios, depending also on the type of substrate to be dyed and its hydrophilicity.
Table 1 shows typical ranges of nominal liquor ratios for each type of machine.
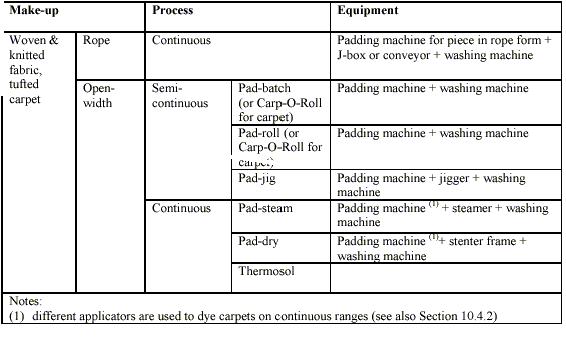
- Continuous and semi-continuous dyeing: (BAT for the Textiles Industry, July 2003)
In continuous and semi-continuous dyeing processes, the dye liquor is applied to the textile either by impregnation (by means of foulards) or by using other application systems. Most commonly, textiles are fed continuously in open width through a dip trough filled with dye liquor. The substrate absorbs an amount of dye solution before leaving the dip trough rollers that control the pick-up of the dye. Surplus stripped dye flows back into the dye bath.
Dye fixation is usually achieved in a subsequent stage using chemicals or heat (steam or dry heat). The final operation is washing, which is usually carried out in washing machinery at the end of the same line. The only difference between continuous and semi-continuous processes is the fact that in semi-continuous dyeing the application of the dye is performed continuously by padding, while fixation and washing are discontinuous.
In continuous and semi-continuous processes the liquor ratio is not of practical importance and it is not used as a parameter. In these processes the factors to be taken into account are the wet pick-up %, i.e. grams of liquor picked up by 100 grams of substrate, and the concentration of the dye.
An overview of the most common techniques and machinery utilised in continuous and semi-continuous processes is given in Table 2.
Table 2 (Literature: IPCC-BAT for Textile Industry, EC 2003)
Typical process parameters
| Process | Temperature [°C] | Heat transfer medium | Residence time | Details | Literature |
| Dry dyeing | 180 °C | rolls | continious | Kratzsch, E. (1976). Konitinuierliche Behandlung zur Entwicklung von textilen Flächengebilden mit latentem Schrumpf, Folge 40. | |
| Wet dyeing | 140 °C | water and chemicals | continious |
4. COMPETITIVE TECHNOLOGIES AND ENERGY SAVING POTENTIALS
- a) Changes in the process
- One-step continuous vat dyeing in pastel to pale shade: (BAT for the Textiles Industry, July 2003)
The conventional pad steam process with vat dyes includes the following steps:
- padding of dyestuff pigments
- intermediate drying
- padding of chemicals/auxiliaries (reducing agents)
- steaming
- oxidising
- washing (several washing and rinsing steps)
In some cases the process can be carried out without steaming and subsequent washing, according to the following simplified sequence (similar to the dyeing process with pigments):
- padding of dyestuffs and chemicals/auxiliaries in one step
- drying
- fixation
Special selected vat dyes with a low tendency to migrate need to be used. Moreover, auxiliaries based on polyglycols and polymers are necessary, which improve pad liquor stability and provide a high fastness level.
Main achieved environmental benefits
A number of steps, in particular the washing operations, are avoided. As a result, only the residual padding liquors have to be disposed of at the end of the process and water consumption is minimised to approximately 0.5 l/kg of textile.
Savings in chemicals and energy are also obtained.
Operational data
A type of recipe for the padding liquor includes:
- binder: 30 – 40 g/l
- sodium sulphate: 5 – 10 g/l
- antimigrant: 10 – 20 g/l
- dyestuff: up to 2.5 g/kg
Among typical process parameters, the pick-up should be as low as possible (50 – 65%) and the liquor temperature should be kept below 35°C. Intermediate drying is carried out at 100 – 140°C, while Thermofixation conditions are typically 30 s at 130°C for cellulose and 30 s at 190°C for polyester/cellulose blends.
- Enzymatic after-soaping in reactive dyeing: (BAT for the Textiles Industry, July 2003)
Dyeing and printing with reactive dyes entails a number of soaping and rinsing steps to remove from the substrate the amount of unreacted and hydrolysed dye. The removal of all unfixed dyestuff from the fibre is essential for obtaining optimum wet fastness, while contributing significantly to energy, water and chemicals consumption of the overall dyeing process.
The suggested technique consists in adopting an enzymatic treatment to remove the non-fixed dyestuff not only from the fibre, but also from the exhausted dye bath. Enzymatic decolourisation of reactive dyestuffs has been proved with Levafix, Remazol, Cibacron, Procion and Synozol [UBA, 2001].
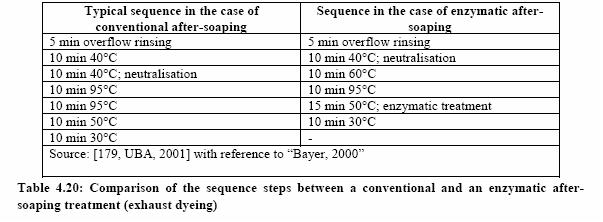
The application of the enzymatic compounds usually takes place in the fourth or fifth rinsing step (see table below):
Main achieved environmental benefits
As Table 4.20 shows, one of the hot rinsing steps can be avoided when using enzymatic after-treatment. Savings in water, energy and detergent consumption are the main advantages achievable with this technique.
Operational data
The enzymatic treatment is carried out as follows (batch process):
- filling with fresh water (50°C)
- addition of a buffer for adjusting the pH
- control of pH (addition of acetic acid, if necessary)
- addition of the enzymatic compound (0.25 g/l)
- running: 10 min
- draining
- pH-controlled dyeing techniques: (BAT for the Textiles Industry, July 2003)
Fibres such as wools, polyamide and silk contain weak acid and weak base groups (e.g. carboxylic and amino functions). Just like the parent amino acids from which all proteins are derived, these fibres show zwitterionic characteristics at pH values close to the isoelectric point (i.e. the pH at which the fibre contains equal numbers of protonate basic and ionised acidic groups).
At a pH below the iso-electric point, the carboxylate anions are progressively neutralised by the adsorption of protons and the fibre acquires a net positive charge (see equate 1):
H3H+ - (fibre) – COO- + H+ = H3N+ - (fibre) – COOH
Conversely, as the pH rises above the isoelectric point, the fibre becomes negatively cahrged as a result of the dissociation of the carboxylic acid groups (eqution 2) and deprotonation of the amino groups by adsorption of hydroxide ions or other anions as shown below in equation 3:
(2) H3N+ - (fibre) – COOH + OH- = H3N+ - (fibre) – COO-+H2O
(3) H3N+-(fibre) – COO- + OH- = H2N – (fibre) – COO- + H2O
Based on these reactions, fibres with zwitterionic characteristics can be dyed by imposing a pH profile at iso-temperature, instead of a temperature profile at iso-pH.
The dyeing process is started in alkaline conditions, above the isoelectric point. At this pH, the carboxylic groups become dissociated and the anionic charged groups repulse anionic dyes. This makes it possible to control the adsorption of the dye on the fibre by gradually decreasing the pH.
At a low enough pH when the number of cationic charges on the fibre increases, the dye becomes attracted to the fibre via coulombic interactions, which provides additional bonding forces that cannot be broken by thermal agitation.
At iso-pH, part of the carboxylic groups are neutralised and at higher temperatures, the dye can move rapidly and with minimal energy through the fibre.
The main difference between temperature- and pH-controlled dyeing is that in the temperature-controlled dyeing the process is controlled by the dye bath exhaustion and thermal migration for the dye, whereas with a pH-controlled profile the dyeing process is controlled by the adsorption of the dye onto the ionic fibre.
The pH profile can be controlled during the dyeing process either by dosing a strong acid or base or by creating a buffer system during the dyeing process (mixture of a weak acid and their conjugates base or vice versa). Two methods are normally used to generate a buffer system. One method is to does a weak acid (e.g. acetic acid) starting from an alkaline bath containing a strong base (or a strong acid starting from a weak base); another method consists in using acid- or base-donors for pH-sliding (ammonium sulphate and hydrolysable organic esters are examples of acid donors).
Main achieved environmental benefits
On of the advantages of iso-thermal dyeing is that the use of specific organic levelling agents or retarders (typically added to the dye bath to allow even dyeing) can be avoided.
Time and energy use with pH-controlled dyeing is lower than with the temperature-controlled process. Energy is saved because the dye bath (and the machine) do(oes) not need to be heated from room temperature up to the migration temperature (above the optimum dyeing temperature). Time is saved because the heating and cooling phases are shorter and no extra time is required for the migration process.
Operational data
As stated earlier, pH steering during batch dyeing can be performed by fitting the machine with dosing systems for acid and alkalis. This is the bets and most effective method because it minimizes the amount of chemicals consumed to shift the pH. However, precise control of the pH profile with this method is difficult as the pH must be measured continuously and the bath must be fully homogenised. This technique is therefore limited to machines where the goods and liquor are well mixed, such as jets and modern carpet winches. Moreover, if a mineral acid (e.g. sulphuric acid) and an alkali are used, the salt content of the dye bath may increase above acceptable levels when recycling water.
Instead of using pH-measuring instrumentation another technique is the generation of a pH-buffer during the dyeing process. In this case, there is no need to measure the pH in a fully contained system. In fact, pH-chemistry and dynamic mass-balancing can predict pH and more importantly, can create a consistent repeatable pH profile. For these reasons this technique, although more expensive (higher consumption of chemicals) and more polluting (higher organic load in the effluent), tends to be preferred by companies in the sector.
The use of decarbonated water is the best way to ensure optimal pH control, especially when weak acid donors are used (when process water is not decarbonated the acid will be consumed in the formation of CO2 rather than for shifting the pH of the bath).
- Energy and water saving: Dyeing machines: (BAT for the Textiles Industry, July 2003)
See the file “Savings - Dyeing machines”
- Avoiding batch softening: (BAT for the Textiles Industry, July 2003)
In batch processing softening agents are often applied after the dyeing process directly in the dyeing machine (e.g. jet, overflow) using the exhaustion method.
Unfortunately, this limits the choice of softening agents to environmentally harmful cationic agents and gives rise to a 10 – 20 % loss of the whole volume of the warm softening bath.
Alternative techniques are the application of softeners by pad mangles or by spraying and foaming application systems. The advantages of these techniques are that the use of cationic softening agents can be avoided and any chemical loss can be reduced to a few percent.
The amount of residual liquors is also reduced compared with the waste water volume produced by a batch process. In this respect, the bets performances are achieved with application techniques such as spraying and foaming, which allow minimum system-losses (residual liquor in the chassis, residual liquor in the pipes and leftovers in the batch storage containers). However, the concentration of active substance is much higher, which makes these liquors not suitable for treatment in a biological system.
Another advantage of applying the softeners in separate equipment after the batch dyeing process is that it is then possible to re-use the dyeing or rinse baths as there is no longer a problem with the presence of residual cationic softeners, which would otherwise limit the adsorption of the dye in the subsequent dyeing process.
Main achieved environmental benefits Savings in water, energy and chemicals are achieved. Less environmentally harmful softening agents are introduced.
- b) Changes in the energy distribution system
- Water re-use/recycling in batch dyeing processes: (BAT for the Textiles Industry, July 2003)
Opportunities to minimise water consumption in dyeing processes may be found in dye bath reconstruction and re-use or re-use of the rinse water for the next dyeing.
Dye bath re-use is the process by which exhausted hot dye baths are analysed for residual colorant and auxiliary concentration, replenished and re-used to dye further batches. Two procedures are possible. With the first method the dye bath is pumped to a holding tank (or to a second identical machine), while the product is rinsed in the same machine in which it was dyed. The dye bath is then returned to the machine for the subsequent batch of material. With the second option, the product is removed from the exhausted dye bath and placed in another machine for rinsing. In this case no holding tank is required, but the material needs additional handling. Dye bath analysis can be performed using spectrophotometer and/or may be determined by production experience based on exhaustion level, volatilisation and dye liquor drag-out.
Since the spent dye bath is usually hot, it is of course convenient to save time and energy by dye bath re-use. However, to assure level dyeing it is normally necessary to start the dyeing process at 50°C. Therefore, the hot spent bath is cooled down and then warmed up again. In some cases this can be avoided. New technologies have been developed which allow dyeing to start at process temperatures. Instead of piloting the temperature one can control the chemical potential of the dye (which is what happens, for example, by adding the sodium hydroxide to reactive dyes). These techniques are suitable for wool dyeing with acid dyes, acrylic dyeing (this would exclude the addition of levelling agents) and for cotton in the case of sulphur dyeing or reactive exhaustion dyeing processes.
The second technique proposed here is similar, but this time the spent rinse bath is re-used to form the next dye bath.
Main environmental benefits
Reduction in water and chemicals consumption; Energy saving (re-use of the hot dye bath) is also possible, in some cases (see above) when dye adsorption is controlled by pH and the bath becomes nearly completely exhausted without cooling down at the end of dyeing.
Operational data
Operational data are reported by the UBA for a plant dyeing PES and wool loose fibre. Wool is dyed with afterchrome or with metal-complex dyes, whereas PES fibre is dyed with disperse dyes. Both dyes are characterised by high exhaustion rates, which allows re-use of the spent dyeing bath for the next batches. All dyeing machines with capacity ranging from 50 to 100 kg (L.R 1:8) have been fitted with holding tanks, temperature and pH control devices and automated dosage systems for formic acid. Most of the holding tanks are constantly used for the same type of dyes and shades (e.g. afterchrome bath for dark shades, etc.). As a result of the improvements, the company has achieved a decrease in specific water consumption from 60 to 25 l/kg.
Another operational experience is reported by ENco for a mill dyeing wool loose fibre. The company operates conical pan type machines and loads the fibre carriers with dry fibre. Mean specific water consumption figures for the conventional dyeing and rinse cycle are 9.5 l/kg and 7.8 l/kg, respectively (1.7 l/kg is retained by the fibre load between dyeing and rinsing). Overall water consumption for a conventional cycle would be 17.3 l/kg.
When re-using the rinse liquor for the next dyeing it is necessary to add on average 1.7 l/kg of fresh water to the dyeing to make up for the water lost when the wet fibre from the previous dyeing is removed. Experience indicates that on average only four cycles of the same shade can be sustained with re-use. Overall water consumption for this four-batch dyeing system is reduced by approximately 33% when compared with the conventional cycle.
- c) Changes in the heat supply system
No information is available.