Phamaceuticals
Back to EFFICIENCY FINDER FOR CHEMICAL INDUSTRY
Back to Information about pharmaceutical ingredients and vitamins
1 Overview
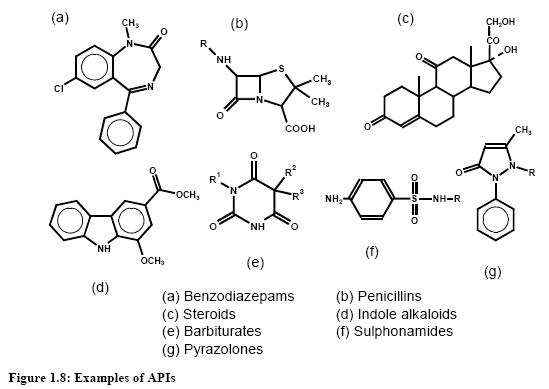
Active Pharmaceutical Ingredients (APIs) are based on organic molecules which have been synthesised and modified to provide medicinal products and comprise the largest segment of available drugs. Biotechnology is part of the pharmaceutical industry today, but drugs based on organic chemistry remain the largest part of R&D and comprise the largest percentage of new drugs launched yearly. Figure 1.8 gives some examples, but in reality the variety in the world is enormous.
2 Production of APIs
- Diazotisation and azo coupling
Diazotisation and coupling processes are important for the manufacture of APIs and represent the essence of azo dye manufacture. Azo dyes are the predominant colourant family, accounting for over 50% of all commercial organic dyes. Diazotisation can also be followed by processes such as hydrazine formation, Sandmeyer reactions and azo double bond reduction. Diazo and coupling components can be halogenated and can contribute to an AOX load in waste water streams. Often, azo coupling includes an immediate metallisation step involving heavy metals to give metal complex dyes.
Chemical reaction
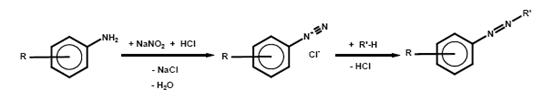
Diazotisation is the reaction of primary arylamines with nitrites, preferably with sodium nitrite, in a usually aqueous mineral acid solution at around 0 °C, whereby the amine is converted into the corresponding diazonium compound. Weakly basic arylamines require a higher acid concentration (NO2- surplus), since diazoamino compounds Ar–N=N–HN–Ar may otherwise form. A further reason for using concentrated acids (e.g. concentrated sulphuric acid) is the fact that diazonium compounds of weakly basic arylamines are readily hydrolysable in dilute acids.
The azo coupling reaction is an electrophilic substitution reaction of the diazonium compound with a coupling component R’H. In order to maintain an optimal reaction sequence, the pH must be kept constant by adding alkalis or buffers.
- Coupling components: phenols, naphthols and amines
- Side reactions: formation of diazo amino compounds decomposition of diazo salts to phenolic compounds formation of isomers processing of isomers contained in the starting material.
Operations
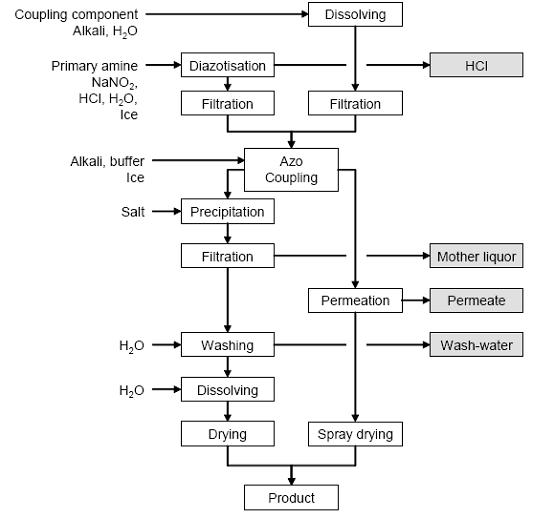
Figure 1 shows a typical sequence of operations for diazotisation and azo coupling in dyestuff manufacturing. Sodium nitrite is added in excess to a solution or suspension of the arylamine (diazo component) in a diazotisation tank. The reaction is cooled to 0 °C by adding ice or by cooling with brine. In a separate tank, the coupling component is dissolved in water and alkali. Both solutions are clarified by filtering and added to the coupling vessel. The addition sequence depends on the particular case, and the precise reaction conditions (pH, temperature) are established by the addition of alkali or ice.
Clarifying may be necessary on completion of the reaction (by filtration over SiO2, Al2O3 or charcoal) to remove unreacted amine and salty, resin-like or oily by-products, followed by precipitation of the product (usually by salting out or pH change), filtration, washing, dissolving and, e.g. spray drying to yield the standardized dyestuff. Alternatively, the reaction mixture is immediately passed through a pressure permeation, followed by, e.g. belt, spin flash, spray or oven drying.
Figure 1: Typical sequence of operations for diazotisation and azo coupling Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
- Esterification
Organic esters are of considerable economic importance. Because of their highly lipophilic and hydrophobic nature and low polarity, esters are widely used as solvents, extractants, and diluents. Ethyl acetate is the most common technical solvent. Large quantities of esters, especially phthalates, adipates, and fatty acid esters, are used as plasticisers. Esters with a pleasant odour are used in fragrances, flavours, cosmetics, and soaps. Esters can be converted into various derivatives and are useful intermediates in the synthesis, e.g. of vitamins or pharmaceuticals.
Chemical reaction
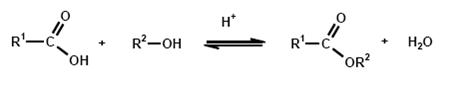
A great variety of production methods for carboxylic acid esters are known, but the simplest and most common method of esterification is the reaction of an alcohol with a carboxylic acid with the elimination of water:
Esterification is the reverse of hydrolysis and leads to an equilibrium reaction, which is the reason that quantitative esterification is possible only by continuous removal of one of the products, i.e. ester or water. In the case of transesterification, an alcohol is released instead of water.
Suitable catalysts are sulphuric acid, hydrogen chloride, arylsulphonic acids such as p-toluenesulphonic acid, and chlorosulphuric acid. Phosphoric acid, polyphosphoric acids, and mixtures of acids are also recommended. If the acids are adsorbed on a solid support, esterification can be carried out as a continuous process.
Removal of water usually involves the addition of entrainers, which form azeotropes with relatively low boiling points and high water contents (usually toluene, xylene, cyclohexane, seldom also benzene or CCl4).
Operations
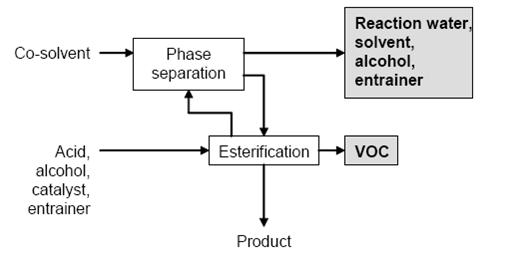
The reaction is generally carried out by refluxing the reaction mixture until all the water has been split off. The water or the ester is removed from the equilibrium by distillation. Water is usually removed by distillation of the azeotrope with the alcohol or an entrainer. After condensation, the azeotrope separates into an aqueous phase and an organic phase, and the entrainer or alcohol is recycled into the reaction mixture. In particular cases, a co-solvent such as benzene or toluene is added to the condensate to achieve separation of the organic phase. Many esters are produced continuously in pipes, distillation columns or plate columns. Ionexchange resins are especially suitable as catalysts in continuous processes. The reactants pass through or over the solid catalyst, and no separation or neutralisation of the catalyst is necessary.
Figure 2: Typical sequence of operations for esterification. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
- Fermentation
The term “fermentation” means process operations that utilise a chemical change induced by a living organism or enzyme, specifically, bacteria, or the micro-organisms occurring in yeast, moulds, or fungi to produce a specified product. Most industrial microbiological processes are enhancements or modifications of metabolic reactions that micro-organisms already carry out. Some applications of fermentation are the production or modification of the U-lactam antibiotics, penicillins and cephalosporins, tetracyclines, and also alkaloids and amino acids.
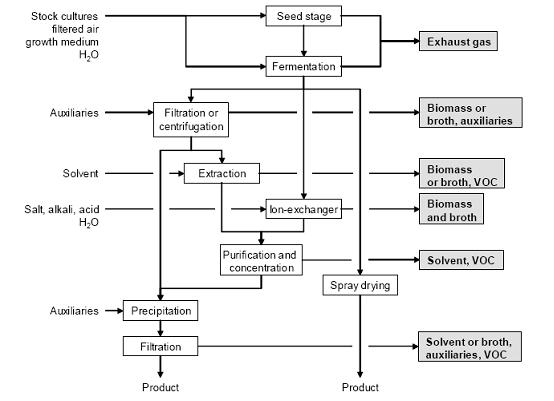
OperationsFigure 1 shows the typical sequences of operations for fermentations, some possible input materials and their associated waste streams.
Raw materials and seed stage
In the large scale fermentation of antibiotics, there are a number of stages, termed “seed stages”, leading to the final production stage. The objective of the seed stages is simply to develop an ever larger and more vigorous population of micro-organisms, with no attempt being made at this stage to produce any antibiotic. Each seed stage is used to innoculate the next, with process times for the individual seed stages usually being less than the final production stage.
The early seed stages are carried out on a laboratory scale and involve the preparation of starter cultures, which are then used to innoculate larger fermenter vessels (from some m3 to 50 m3 or more) containing a sterile medium.
The raw materials used as the growth medium in the fermentation process are primarily liquids stored in bulk, for example corn steep liquor, rapeseed oil and starch hydrolysate. These types of raw materials are non-volatile and there are no special precautions required for the transfer and batching into fermenter vessels. Bulk storage tanks for these materials are usually provided with secondary containment and high level alarms to prevent overfilling. Other batched solid raw materials are dispensed from bags and are dosed into the fermenter medium at low levels. The batching area is provided with an air extraction system for the protection of the operators, with the extracted air passing to a dust scrubber before being released to the air. Alternatively, the batching area is designed with closed systems, which are dust free and therefore not hazardous to the operators. Equipment and growth medium are sterilised above 120°C for 20 minutes.
Fermentation stage
The fermentation stage is carried out in a large stirred fermenter (from some m3 to 200m3 or more) and is an aerated, batch-fed process. The batched medium is designed to support only a limited amount of further growth and is steam-sterilised within the fermenter. After sterilisation, it is inoculated with the final seed stage broth. Further sterilised nutrients are added continuously (“fed”) during the fermentation in such a way that the growth of the microorganism is precisely controlled and the conditions made favourable for antibiotic production. The process lasts up to eight days.
Figure 3: Typical sequence of operations for fermentation and downstream work-up. Possible input materials (on the left) and associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
Product work-up
The following work-up steps depend on the properties and location of the product. The products are obtained by separation of the biomass from the broth by:
- filtration (conventional or ultrafiltration) and extraction of the filtered broth with an organic solvent and pH adjustment (e.g. penicillin G) or extraction of the biomass with organic solvents (e.g. steroids)
- filtration (conventional or ultrafiltration) and product precipitation from the filtered broth by adjusting the pH and/or by the addition of auxiliaries (e.g. tetracyclines)
- pH adjustment and processing of the unfiltered broth over an ion-exchanger (alkaloids, amino acids)
- direct spray drying of the unfiltered broth (e.g. for feed industry purposes).
Intracellular products need an additional mechanical step of cell destruction before extraction.
Further steps can also be carried out in order to optimise the purity or concentration. The choice of methods are:
- evaporation
- ultrafiltration
- chromatography and/or ion-exchange
- reverse osmosis.
After purification, the product is obtained by conventional crystallisation and drying.
- Halogenation
Halogenation is one of the most important and versatile processes in chemistry. The industrial application is dominated by chlorinations, due to the different reactivity and the higher price for bromine, iodine and fluorine.
Side chain chlorinated alkyl aromatics, particularly those based on toluene and xylene, as well as nucleus halogenated aromatics, have an exceptional place in organic fine chemistry, because of their role as chemical intermediates in the manufacture of chemical products of almost all kinds, including dyes, plastics, pharmaceuticals, flavours and fragrances, pesticides, catalysts and inhibitors.
Bromination is a key process in anthraquinone chemistry and the manufacture of organic flameretardants.
Heavily halogenated aromatic hydrocarbons Especially as a result of the environmental persistence of the heavily chlorinated benzenes, toluenes and biphenyls, in recent years drastic measures have been applied to this range of chemicals, such as prohibitions, and restrictions on their production and use, and legislation regulating waste disposal. Possible side reactions of the chlorination process can result in the formation of polychlorinated biphenyls or hexachlorobenzene. The combustion of aromatics containing chlorine can lead to the formation of polychlorodibenzo dioxins/-furans (PCDD/PCDF).
Chemical reaction
These chemicals are of major relevance on an industrial scale in substitutions of the aromatic nucleus and in the substitution of aliphates. In both cases, hydrogen is replaced by halogen (X) and the related hydrogen halide is created:
R – H + X2 R – X + HX Ar – H + X2 Ar – X + HX
Both reactions are exothermic but the aliphate substitution follows a radical chain mechanism, initialised by ultraviolet light (irradiation with mercury vapour lamps), while the halogenation of the aromatic nucleus is based on an electrophilic mechanism supported by Friedel-Crafts catalysts (i.e. Lewis acids such as FeCl3, AlCl3 …).
Generally, a mixture of isomers and/or compounds with a different degree of halogenation is obtained and side reactions following alternative mechanisms cannot be completely suppressed. The product mix depends on the aromatic/halogen ratio, the reaction conditions and the choice of the catalyst.
A wide range of organic and aqueous solvents are currently in use, and especially tetrachloromethane, tetrachloroethane, dichlorobenzene and trichlorobenzene are recommended for halogenations.
Bromine is more efficiently used in aromatic substitution reactions if it is generated in situ from hydrogen bromide using chlorine: ArH + HBr + Cl2 ArBr + 2 HCl
Another approach is the use of an alcohol as the solvent to co-produce an economically useful alkyl bromide, by the reaction of by-product HBr with the alcohol. Methanol is the solvent of choice since the resulting methyl bromide can be widely marketed as a fumigant.
Operations
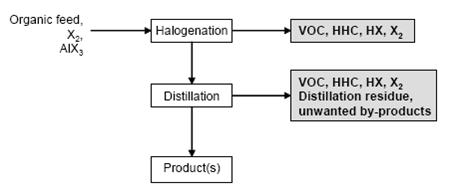
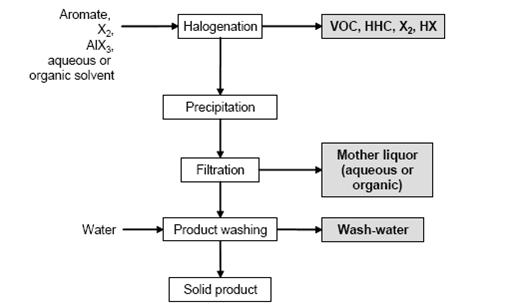
Figure 4 shows a typical sequence of operations for the halogenation to distillable products. Figure 5 shows a typical sequence of operations for the halogenation precipitation of the product.
Figure 4: Typical sequence of operations for the halogenation to distillable products Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
Figure 5: Typical sequence of operations for halogenation with precipitation of the products Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
In a typical batch reaction, the halogen is added to the stirred aromatic or a stirred aromatic solution. The reactor material depends on the reactants and the chosen reaction mechanism. The exothermic reaction is controlled by the rate of halogen addition, which is dependent on the refrigeration capacity of the reactor cooling system. The choice of temperature profile is based on the reactivity of the aromatic. On completion of the reaction, degassing is carried out with nitrogen. The product is distilled or precipitated (e.g. by cooling or water addition) and the resulting slurry is filtered, washed and dried. Most side chain chlorinations are carried out continuously or discontinuously in bubble column reactors of enamel or glass, e.g. of the loop type. The reactor is filled with the starting material, heated to at least 80 °C and chlorine is introduced until the desired degree of chlorination is reached. The reaction is stopped by the introduction of N2. Products of different degrees of chlorination are separated by distillation to be directly marketed, hydrolised to give the related benzaldehydes or benzoic acids/benzoyl chlorides, or are used for further chlorination.
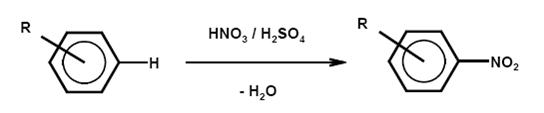
- Nitration
Liquid phase nitration is a dominant step in the manufacture of common high explosives and important for the production of a wide range of aromatic intermediates for dyes, agrochemicals, pharmaceuticals or other fine chemicals. A typical nitration reaction is higly exothermic, therefore, for a safe mode of reaction, a dosage controlled process with precautions securing no accumulation of reactants is necessary. Typical nitroaromatic production is based on high yield processes, with more than 80 % of the total cost being the cost of the raw materials. Integral requirements of all efficient nitration processes are sulphuric acid regeneration and isomer control and separation. Nitration of the important naphthalene mono- and disulphonic acids is usually performed with the formed sulphonated mass. Among the typical raw materials are halogenated aromatics, which can contribute to the AOX load of waste water streams.
Chemical reactionNitration is the irreversible introduction of one or more nitro groups into an aromatic system by electrophilic substitution of a hydrogen atom. O-nitration to give nitrates and N-nitration to give nitramines are far less important for aromatic compounds but relevant for the manufacture of explosives.
Nitration is normally carried out in a liquid phase reaction with a mixture of nitric and sulphuric acids (mixed acid) and occasionally with nitric acid. A typical mixed acid, for example for mononitration, consists of 20 % nitric acid, 60 % sulphuric acid and 20 % water (this is referred to as 20/60/20 mixed acid). The strength of the mixed acid and the temperature can be varied to maximise the formation of the required isomer. Stronger mixed acid and higher temperature lead to oxidative side reactions. An important side reaction leads to phenolic by-products.
Operations
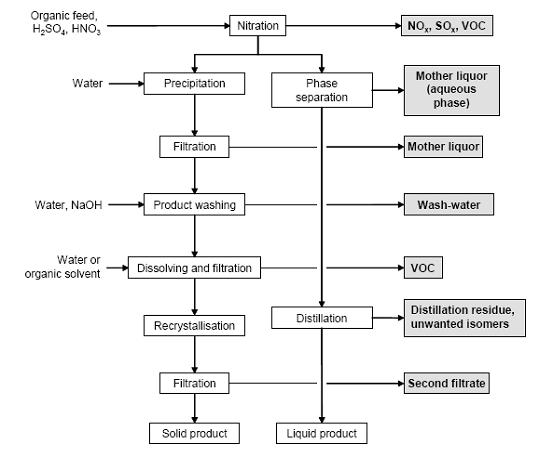
Figure 6 shows a typical sequence of operations for the nitration of aromatic compounds, possible input materials and associated waste streams. The reaction is carried out in cast iron, stainless steel or enamel-lined mild steel reactors. Temperatures vary normally between 25 and 100 °C. The substrate is dissolved in the sulphuric acid phase and the mixed acid is subsequently added. On completion of the reaction, the batch is transferred into water to give a two phase mixture of diluted acid and an organic product phase. After phase separation, liquid products are purified by distillation. The remaining acid phase can be extracted with the feed material in order to recover organic compounds. Solid products are crystallised (where necessary, by the addition of cold water). The crude nitroaromatic is washed with water and diluted NaOH to remove the acids and phenolic by-products. Depending on the quality requirements, a recrystallisation from water or organic solvent may be necessary. Isomer separation is carried out within the crystallisation, washing or distillation steps.
Figure 6: Typical sequence of operations for a nitration. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
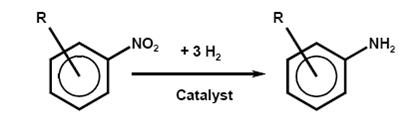
- Reduction of aromatic nitro compounds
One of the most industrially important reduction processes in industrial use is the conversion of an aromatic nitro or dinitro compound into an arylamine or arylene diamine. Aromatic amines are widely used as dye intermediates, especially for azo dyes, pigments, and optical brighteners; as intermediates for photographic chemicals, pharmaceuticals, and agricultural chemicals; in polymers via isocyanates for polyurethanes; and as antioxidants.
Among reduction methods, there are three of major relevance in organic fine chemistry:
- catalytic hydrogenation, which is extremely important industrially because of its universal applicability; most processes can be carried out successfully by catalytic hydrogenation
- Béchamp and Brinmeyr reduction with iron, which is the classical method
- alkali sulphide reduction, which is the selective method in specific cases, such as in the manufacture of nitroamines from dinitro compounds, the reduction of nitrophenols, the reduction of nitroanthraquinones and the manufacture of aminoazo compounds from the corresponding nitroazo derivative.
All three methods are also applied to halogenated nitro compounds, and can thus contribute to AOX loads in waste water streams.
- a) Catalytic reduction with hydrogen
Chemical reaction
The catalytic reduction of the nitro compounds is very exothermic. To reduce these hazards, the concentration of nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst, are controlled.
Most aromatic nitro compounds are hydrogenated in the liquid phase. In this case, the pressure and temperature can be changed independently. The temperature is limited by the hydrogenation reaction of the aromatic ring which occurs above 170–200°C.
Normally, the reduction is carried out at 100–170°C. Sensitive compounds are hydrogenated at lower temperatures (20–70°C) or at lower pressures (1–50 bar). 1–50 bar are used normally.
The preferred solvents are methanol and 2-propanol; and also dioxane, tetrahydrofuran, and N-methylpyrrolidone are used. In the hydrogenation with a water immiscible solvent, such as toluene, the water must be removed, as in solvent-free hydrogenation, in order to maintain the activity of the catalyst. If the amine has a good water solubility, water is used as the solvent. Water also can be used in cases where the nitro compound forms water-soluble salts with alkalis, such as with nitrocarbonic or sulphonic acids. In practice, only Raney nickel, Raney nickel-iron, Raney cobalt, and Raney copper are used as pure metal catalysts because of their relatively low cost. Precious metal catalysts, such as Pt and Pd, are generally used at concentrations of 0.5 – 5 wt-% on support material with large surfaces, such as charcoal, silica, aluminium oxide, or alkaline-earth carbonates.
Process hazards
The catalytic reduction of nitro compounds is very exothermic. Unless this heat is dissipated properly, decomposition and even explosions can result, especially if the thermal decomposition of the nitro compound occurs or if condensation reactions are initiated as may be the case with chloro-nitro compounds. The industrial hydrogenation of aromatic polynitro compounds in the liquid phase without solvents especially requires precautions. To reduce these hazards, the concentration of the nitro compound, the amount and partial pressure of the hydrogen, the temperature, and the activity of the catalyst are controlled. The nitro compound is continuously added in small quantities, thus keeping its concentration below 2 %. De-ionised water is added to remove the heat of the reaction by continuous evaporation and to slow down the activity of the catalyst.
Operations
The vast majority of aromatic amines have small annual volumes (<500 tonnes) and are produced by batch hydrogenation with catalyst slurries. The reaction is carried out in stirred, steel or stainless steel autoclaves or in loop reactors. Loop reactors show increased heat and mass transfers and improved reaction selectivity, shorter batch cycle times and higher product yields. In addition, catalyst usage is often lower. The addition sequence depends on the particular reactants. On completion the reaction mass is cooled and the catalyst is removed by filtration.
- b) Reduction with iron
Chemical reaction
The reduction of nitroaromatics is carried out in the presence of small amounts of acid (HCl, H2SO4, HCOOH, CH3COOH) as shown in the following equation:
4 Ar – NO2 + 9 Fe + 4 H2O 4 Ar – NH2 + 3 Fe3O4
The acid is used for the activation of the iron. Only 2 – 3 % of the hydrogen is derived from the acid but 97 – 98 % comes from the water.
Operations
Normally the nitroaromatic is added to the mixture of iron/water/acid (excess of iron about 15-50 %) often in the presence of an organic solvent (toluene, xylol, alcohols) and the mixture is heated to reflux. Depending on the reactivity of the aromatic, other addition sequences may be required. In some cases the acid is omitted (neutral iron reduction). The build-up of unreduced excess nitro compound must be avoided and the final mixture should be tested for its total absence. After basification with soda ash (anhydrous sodium carbonate) to precipitate soluble iron, the iron compounds are removed by filtration.
- c) Alkali sulphide reduction
Chemical reaction
The alkali sulphide reduction is a mild and selective reaction according to the following equation, without strict stoichiometry:
Ar – NO2 + Na2S2 + H2O Ar – NH2 + Na2S2O3
Other reducing agents in use are Na2S or NaSH, which also form Na2S2O3. Sulphur may be added to reduce the required amount of sulphide.
Operations
Dilute aqueous sulphide is added to the solution or emulsion of the nitro compound. Temperatures (in the range of 80–100°C) and concentrations depend on the reactivity of the nitroaromatic. An excess of sulphide is avoided in the case of the selective reduction of polynitro compounds.
PRODUCT WORK-UP:
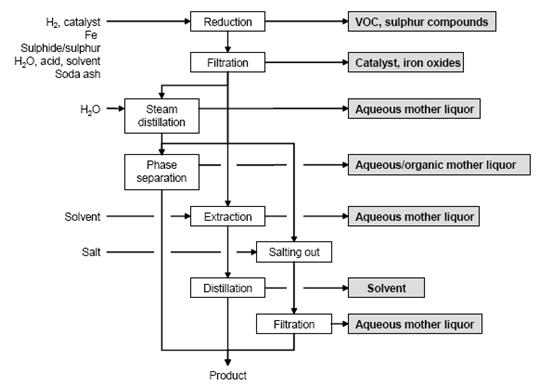
Figure 7 shows a typical sequence of operations for the reduction of aromatic nitro compounds, possible input materials and associated waste streams. The work-up depends on the properties of the amine obtained. Common methods are:
- separation as a liquid
- cooling and salting out
- steam distillation
- extraction with organic solvent, and
- pH adjustment if necessary.
Figure 7: Typical sequence of operations for the reduction of an aromatic nitro compound. Possible input materials (on the left) and the associated waste streams (grey background), BREF on Organic Fine Chemicals, Aug. 2006
4 Legal requirements and process modifications
Where API manufacture on a site requires the observance of the rules of current Good Manufacturing Practice (cGMP) or approval by the European Medicine Evaluation Agency (EMEA), the United States Food and Drug Administration (FDA) or other applicable medicine approval authorities, process modifications can be only carried out fulfilling the required variation procedure. This represents a serious obstacle for the redesign of existing processes. This is even more the case if the API is supplied to a number of different marketing application holders (which is the case for about 75% of the total volume of API production).
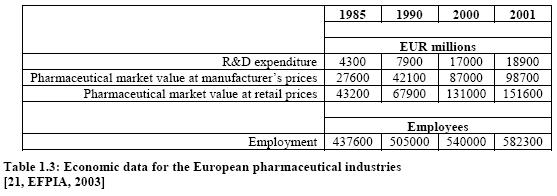
5 Economics
The pharmaceutical industry is a major industrial asset to the European economy, strongly research-based and one of the best performing high technology sectors. Europe produces more than 40 % of the world’s pharmaceutical output by value, making it still the world’s leading manufacturing location ahead of the US (over 30 %) and Japan (20 %).
Although Europe still leads the world in pharmaceutical manufacturing, the US has taken the lead in innovation in terms of R&D investments and the introduction of new pharmaceuticals, e.g. patenting biomedicines. Like many other industries, the pharmaceutical industry is undergoing change. In addition to constantly assimilating new technologies into its research and adjusting to changing market and regulatory environments, a number of pharmaceutical company mergers are taking place. The pharmaceutical industry is highly fragmented. The largest companies have less than 5 % of the worldwide market share for pharmaceuticals. Perhaps as a result, mergers and acquisitions have become more frequent. Some examples are the merger of the two British companies Glaxo and Wellcome; the merger of the life sciences operations of Hoechst, Marion Merril Dow, Rousell, and Rorer in a series of transactions to form Aventis; Sanofi merged with Synthelabo; Novartis which was formed by a merger of the Swiss companies Ciba Geigy and Sandoz; and the merger of Astra and Zeneca to form AstraZeneca.
Literature: BAT for the Manufacture of organic fine chemicals, August 2006
Back to EFFICIENCY FINDER for chemical industry
Back to Information about pharmaceutical ingredients and vitamins