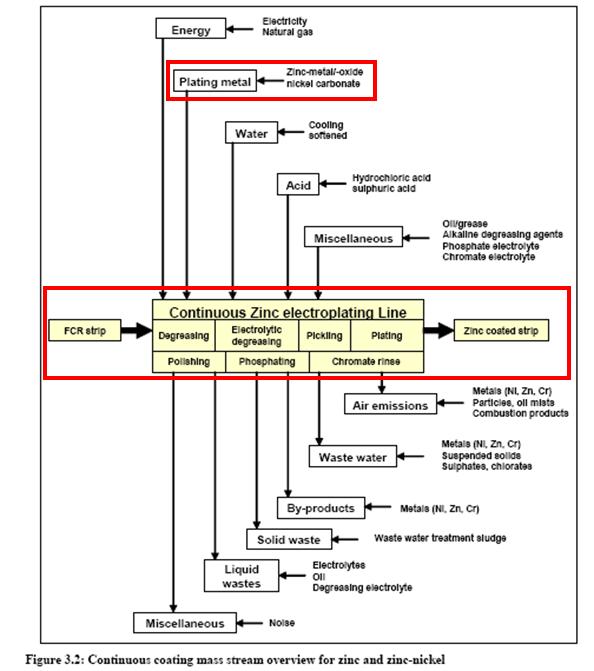
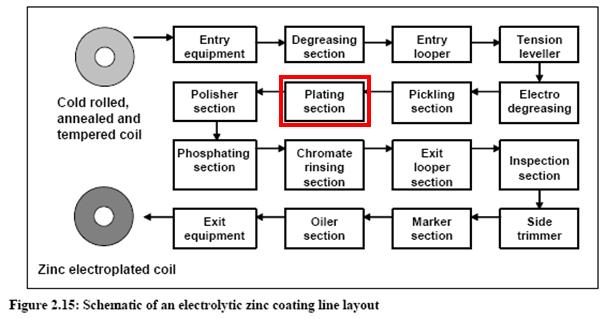
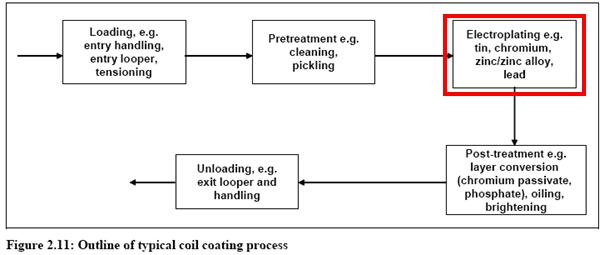
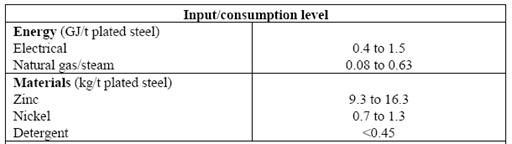
Plating zinc and zinc alloy in metal industry
From Efficiency Finder
Jump to navigationJump to search
Back to EFFICIENCY FINDER FOR METAL INDUSTRY
- Plating zinc and zinc alloy flowsheet
Literature: BAT for the Surface Treatment of Metals & Plastics, 2006
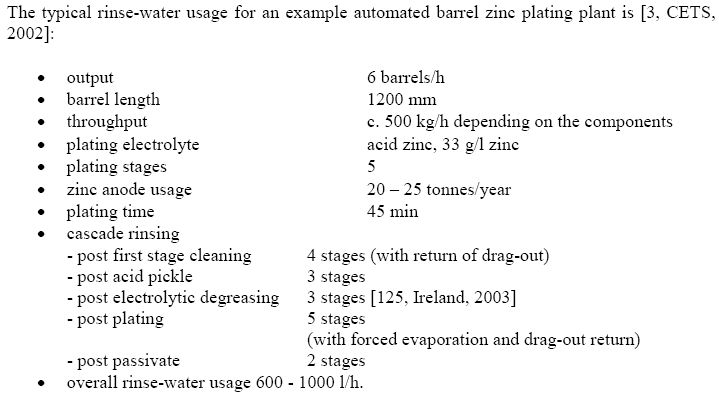
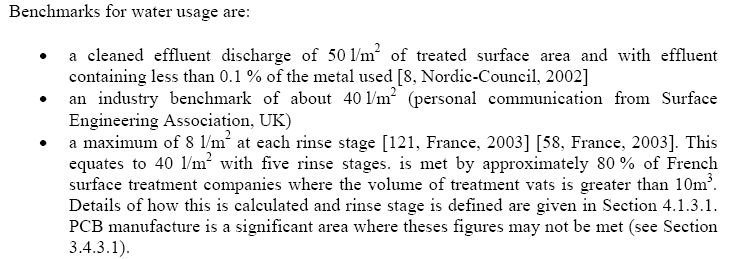
- Typical parameters of the process
| Process | Temperature [°C] | Heat transfer medium | Residence time | Chemicals | Concentration | Details | Literature |
| Plating with alcine cyanide zinc solutions | Zinc oxide, sodium hydroxide, sodium cyanide | Zinc oxide: 10-30 g/l; sodium hydroxide: 80-120 g/l; sodium cyanide: 5-100 g/l | BAT for the surface treatment of metals and plastics, August 2006 | ||||
| Plating with alkaline cyanide-free zinc solutions | Zinc oxide, sodium hydroxide, potassium hydroxide | Zinc oxide: 5-15 g zinc/l; sodium hydroxide or potassium hydroxide: 100-150 g/l; | |||||
| Plating with acid zinc solutions | Alloys: zinc/iron(<1%) from alkaline cyanide-free electrolytes; zinc-cobalt(<3% Co) from acid or cyanide-free electrolytes; zinc-nickel(<15% Ni) from acid (ammonium chloride-based) or cyanide-free electrolytes |
- Plating zinc and zinc alloy
Literature: BAT for the Surface Treatment of Metals & Plastics, 2006
Literature: BAT for the Surface Treatment of Metals & Plastics, 2006
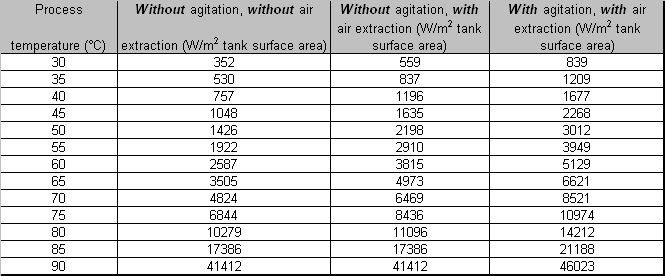
- Energy losses from the surface area of heated process solutions
LITERATURE: BAT for the Surface Treatment of Metals and Plastics, May 2005
Zinc electroplating:
LITERATURE: BAT for the Surface Treatment of Metals and Plastics, May 2005