Polyamides
Back to Information about polyamides
1 General description
Polyamides are chemically characterised by a macromolecular structure having the amide group (-NH-CO-) which is formed by the reaction of a carboxylic group with an amino group as a recurring functional unit that gives the specific chemical properties to the final products. Linear polyamides, widely known as ‘nylons’, from the original DuPont trademark name, are the most common category of the family. Generally, there are two different chemical ways to form the amide group. Therefore, linear polyamides are divided in two groups:
- AB type.
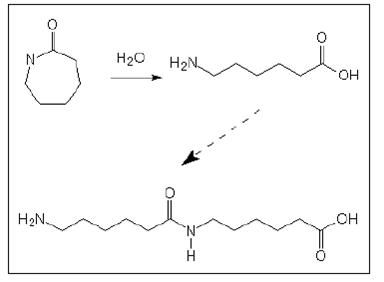
These are produced by polymerisation of lactams or e-amino acids, where A indicates the amino group and B the carboxylic group and both are part of the same monomer molecule. The most important product of this group is polyamide 6 (PA 6) where ‘6’ indicates the number of carbon atoms in the original monomer; in this case f-caprolactam. Other polyamides of this group are polyamide 11 and polyamide 12. The basic reaction, ring opening and polyaddition of f-caprolactam is shown in the following Figure.
Figure 1: Basic reaction of AB type polyamides
- AA-BB type.
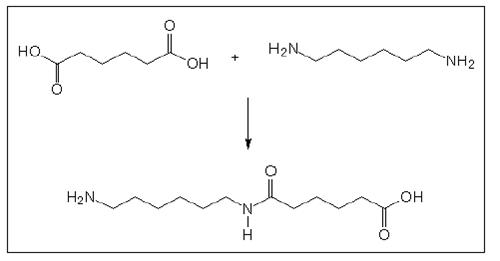
Polyamides of the AA-BB type are produced by the polymerisation of a diamine, indicated by AA, and dicarboxylic acid, indicated by BB. Polyamide 66 is the mostly produced product of this type. ‘66’ in this case indicates 6 carbon atoms between the two amino groups of the diamine and 6 carbon atoms of the dicarboxylic acid. The basic reaction 1.6 – hexanediamine and adipic acid is shown in the following Figure.
Figure 2: Basic reaction of AA-BB type polyamides
Nylons were the first synthetic semi-crystalline plastics, the first synthetic fibres and the first engineering plastics. Today, polyamides are used for various different applications; an overview of the most important ones is given in the following Figure.
File:Main applications for polyamides.jpg
Figure 3: Main applications for polyamides
Polyamides can be easily moulded. They are hard and brittle and resistant to abrasion, shrinkage and heat. Certain polyamides are especially flexible and impervious to impact. Polyamides are resistant to deterioration by alkalis, petroleum products, and organic solvents. Hot phenol, formaldehydes, ultraviolet light and mineral acids destroy polyamides. Most polyamides are self-extinguishing in the event of fire.
2 Flow diagram of ESBR production
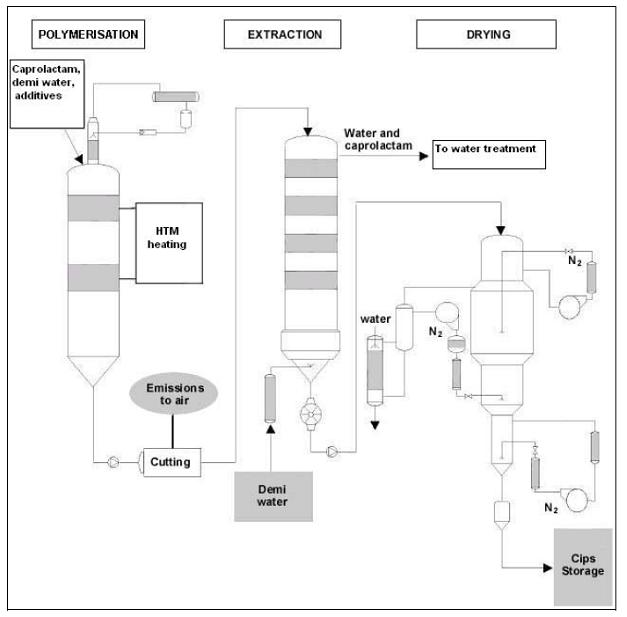
- Continuous polymerisation of PA 6
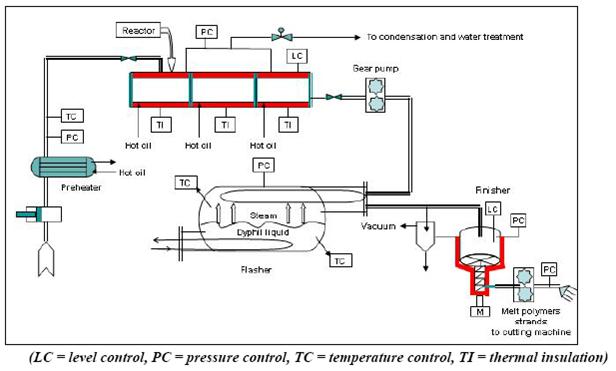
Figure 2: Flow diagram of the continuous PA 6 process
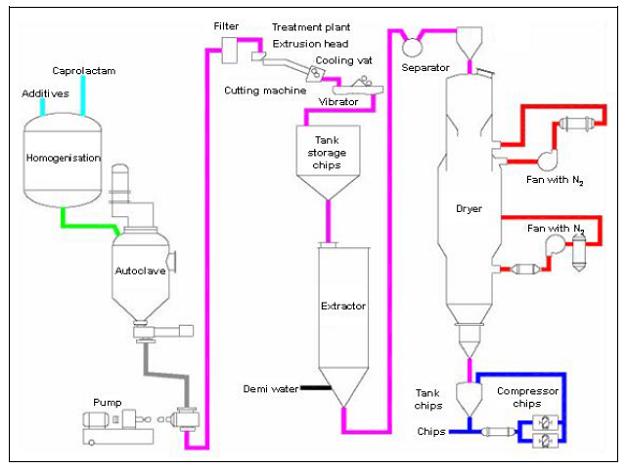
- Discontinuous polymerisation of PA 6
Figure 3: Flow diagram of the discontinuous PA 6 process
- Continuous polymerisation of PA 66
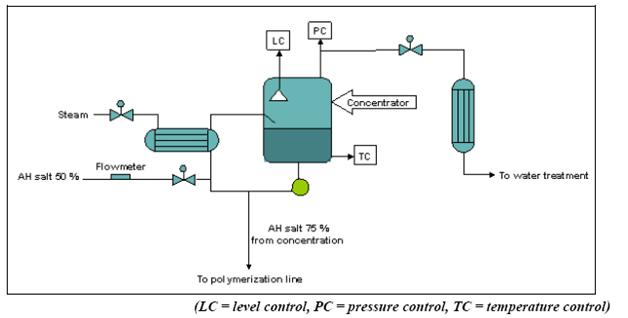
Figure 4: Flow diagram of the salt concentration process for PA 66 production
Figure 5: Flow diagram of continuous PA 66 process
- Batch polymerisation of PA 66
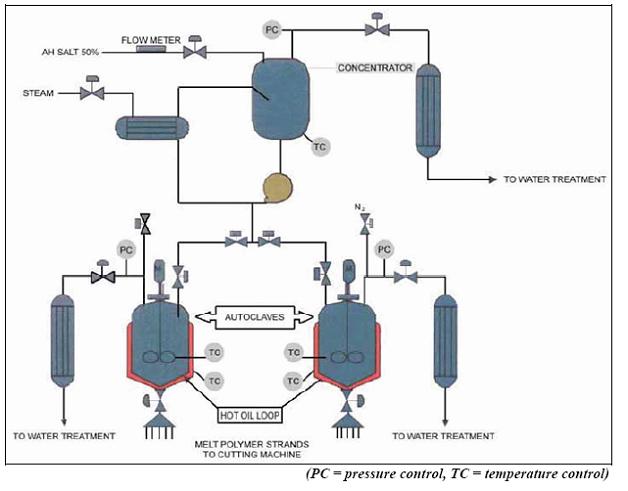
Figure 6: Flow diagram of the batch PA 66 polycondensation process
3 Emission and consumption of polyamide plants
[[Image:Emissions and consumptions from polyamide production processes.jpg
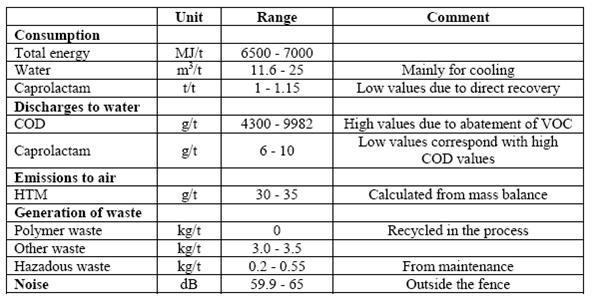
Table 1: Emissions and consumptions from polyamide production processes
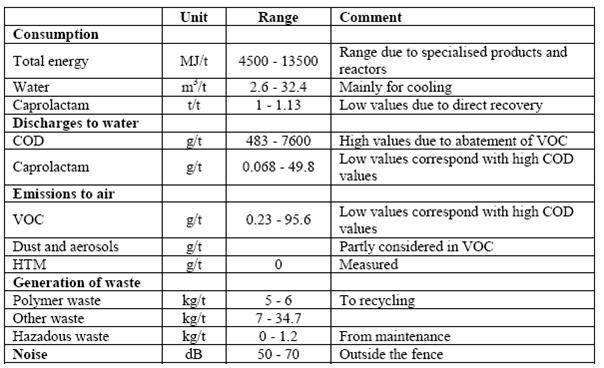
Table 2: Emission and consumption data from the continuous PA6 production process
Table 3: Emission and consumption data from the batch PA6 production process
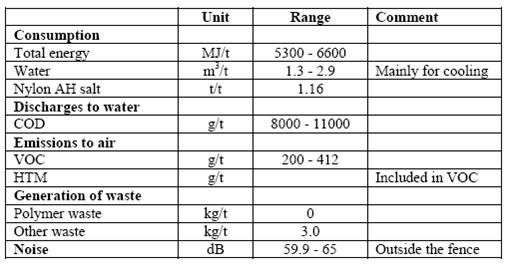
Table 4: Emission and consumption data from the continuous PA66 production process
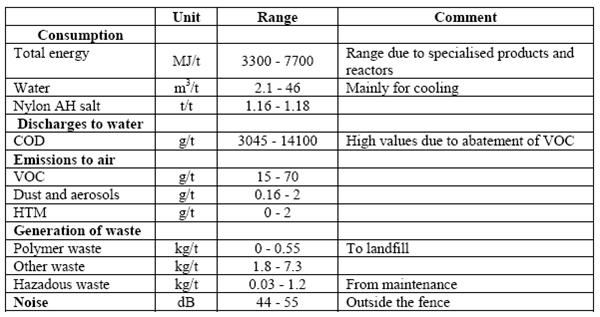
Table 5: Emission and consumption data from the batch PA66 production process
Literature: BAT for Polymers, October 2006
Back to Information about polyamides